Microautophagy is essential for preventing aging
Researchers from Osaka University have shown for the first time that damaged lysosomes are repaired by a process called microautophagy, which is essential for preventing aging
To age or not to age! How does aging affect organisms on a cellular level? What mechanisms help cells survive self-inflicted or external harm? It is known that lysosomes—critically important cellular structures—are crucial for digesting damaged cellular components and pathogens, and maintain stability within cells and tissues. But can they also be repaired, and if so, how?
In a study published this month in EMBO Reports, researchers from Osaka University and Nara Medical University have shown that damaged lysosomes are repaired by a mechanism called “microautophagy” and have identified two key regulators of this process.
Microautophagy is one of the three main types of autophagy in most higher organisms. It is a regulated process by which cellular components that have become dysfunctional or are no longer required are broken down. Although it is assumed to be involved in defense mechanisms collectively called lysosomal damage responses, the details remain unknown.
Lysosomes frequently become damaged and lysosomal dysfunction has been linked to accelerated aging and a shortened lifespan. In this study, the researchers tried to understand the repair mechanisms. To identify a novel regulator of lysosomal damage response, they focused a signaling pathway called Hippo pathway which controls multiple processes such as cellular growth.
They knocked down individual components of the Hippo pathway in the human cells, and then observed whether the cells could respond to induced lysosomal damage. This screening revealed that a protein called Serine-threonine kinase 38 (STK38) is essential for the lysosomal damage response.
They then found that STK38 works with a protein complex called the “endosomal sorting complex required for transport (ESCRT) machinery”, which was already known to be linked to lysosomal repair. “STK38 recruits the protein ‘vacuolar protein sorting 4’ (VPS4) to damaged lysosomes and is crucial for disassembling the ESCRT machinery at the end of the repair process,” explains lead author of the study Monami Ogura. They further found that lysosomal membrane repair by ESCRT machinery is mediated by microautophagy.
They also identified that non-canonical lipidation of a subfamily of autophagy-related protein 8 (ATG8s) molecules—the key autophagy proteins—known as Gamma-aminobutyric acid receptor-associated proteins (GABARAPs) is required for this process. Lipidation, the process of modifying ATG8s with lipid extensions, is the main process involved in autophagy. In non-canonical lipidation ATG8s are lipidated into single-membrane endolysosomes, instead of double-membrane phagophore seen in canonical lipidation.
The researchers showed that the GABARAPs are essential for the first step of the process of lysosomal repair. “We showed that non-canonical lipidation of ATG8s is crucial for the initial recruitment of the ESCRT machinery to damaged lysosomes and their subsequent repair,” explains senior author Shuhei Nakamura.
The team showed that depletion of the regulators of microautophagy increased the rate of senescent cells and shortened lifespan in C. elegans. Both STK38 and GABARAPs also have evolutionarily conserved roles, indicating the significance of this pathway in maintaining lysosomal integrity, healthy cellular function, and the prevention of cellular senescence and organismal aging. The detailed understanding provided by this study paves the way for increasing healthy aging and has great therapeutic value for the treatment of age-related diseases.
Fig.1
Overview: Lysosomes are repaired by ESCRT-driven microautophagy, and STK38 and GABARAPs are key regulators of this process by recruiting ESCRTs to lysosomes. These regulators are essential to maintain lysosomal integrity and prevent aging.
Credit: Osaka University
Fig. 2
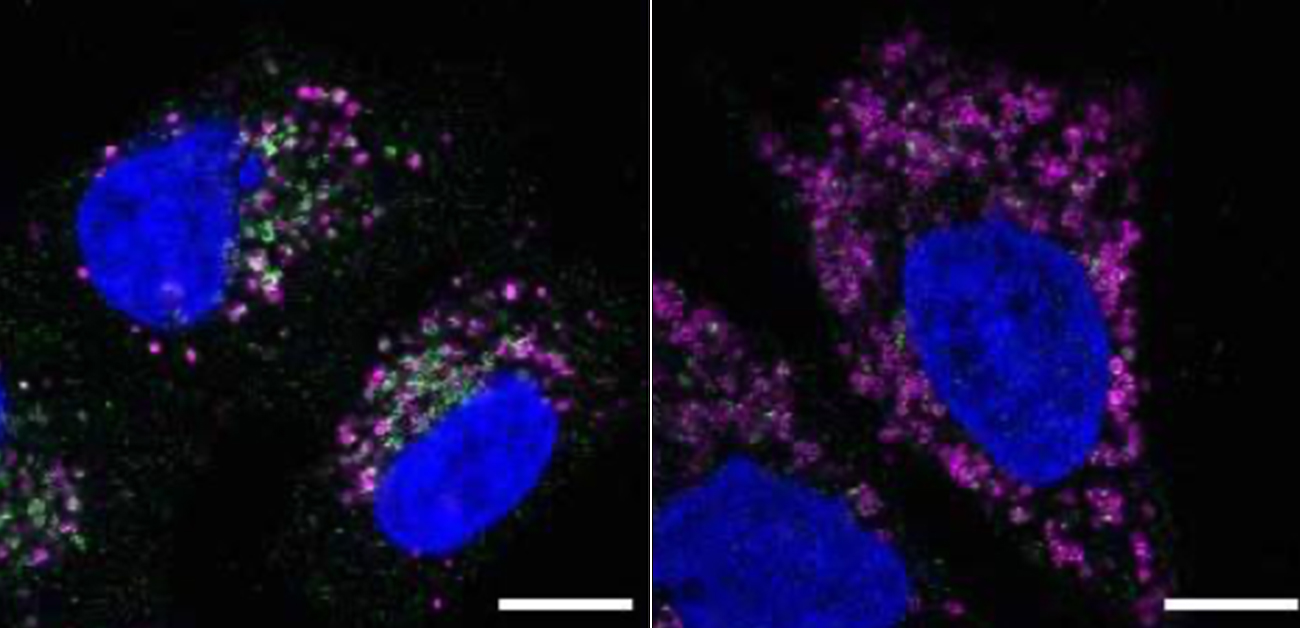
STK38 is required for lysosomal recruitment of VPS4.: In control cells, VPS4 (green) colocalizes with lysosomes (magenta) on conditions of lysosomal damage (lower left). However, formation of VPS4 puncta on lysosomes was suppressed in STK38-knockdown cells (lower right).
Credit: Modified from Ogura et al., EMBO Rep, 2023
Fig. 3
Depletion of STK38 accelerates aging.;(Left panel) Accumulation of damaged lysosomes (green) is increased in STK38-KO C. elegans.
(Right panel) Lifespan is curtailed in STK38-KO worms (red line) compared with wild type (black line).
Credit: Modified from Ogura et al., EMBO Rep, 2023
The article, “Microautophagy regulated by STK38 and GABARAPs is essential to repair lysosomes and prevent aging”, was published in EMBO reports at DOI:10.15252/embr.202357300.
