Mechanism behind autophagy trigger unveiled
A research team led by Osaka University has uncovered a novel mechanism essential for initiating autophagy.
An international research team led by Osaka University has identified a new mechanism crucial for the initiation of autophagy, a self-degradation process cells use to eliminate unneeded or damaged components. In recent years, autophagy has also been recognized for its roles in aging and lifespan regulation.
During autophagy, intracellular molecules and structures are sequestered within a membrane-bound structure known as an autophagosome, which is subsequently degraded in lysosomes. It is well-established that the formation of autophagosomes involves the coordinated action of multiple autophagy-related proteins.
Previously, the research group revealed that autophagosome formation takes place on the endoplasmic reticulum membrane in close proximity to mitochondria within cells. They also discovered that the PI3K complex, an autophagy-related protein, is essential for this formation process. The activity of the PI3K complex is controlled by the ULK1 complex.
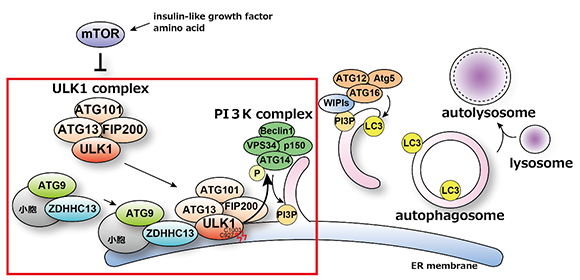
The ULK1 complex is known to translocate from the cytoplasm to the endoplasmic reticulum membrane, where autophagosomes are formed at the onset of autophagy. However, the underlying mechanism and significance of this process were not fully understood until now. In a recent article published in Nature Communications, the research team revealed that the palmitoylation of ULK1 triggers a series of reactions that initiate autophagy.
The team identified ZDHHC13, a palmitoylation enzyme, as a key player in this process through their search for intracellular factors involved in the initiation of autophagy.
Mutations of ZDHHC13 have been linked to various diseases, including Huntington’s disease, while autophagy is also implicated in the development and progression of conditions such as cancer and neurodegenerative disorders.
Senior author Maho Hamasaki explained, “Our team discovered that ZDHHC13 palmitoylates ULK1, thereby localizing the ULK1 complex to autophagosome formation sites. This palmitoylation is also involved in the phosphorylation of the ATG14L protein within the PI3K complex, which in turn regulates the activity of the PI3K complex.”
Understanding the molecular mechanisms that initiate autophagy is expected to enhance our knowledge of autophagy-related diseases.
Lead author Keisuke Tabata added, “Autophagy not only provides a nutrient source through intracellular degradation but also plays a crucial role in maintaining normal cellular function and preventing various pathologies. We will continue to investigate how autophagy begins, furthering our understanding of the mechanisms behind related diseases.”
Fig. Upon autophagy induction, ZDHHC13 is recruited to autophagosome formation sites together with ATG9A. ULK1 is palmitoylated at Cys927 and Cys1003 residues by ZDHHC13, and the ULK1 complex are anchored to an autophagosome formation site. The palmitoylation of ULK1 promotes phosphorylation of ATG14L which leads VPS34 activation. Activated PI3-kinase complex produces PI3P at autophagosome formation sites. These sequential reactions trigger efficient autophagy induction.
Credit: Keisuke Tabata
The article, “Palmitoylation of ULK1 by ZDHHC13 plays a crucial role in autophagy,” was published in Nature Communications at DOI: https://doi.org/10.1038/s41467-024-51402-w.

