Furthering fat loss in the fasting response
Researchers led by Osaka University explore the relationship between autophagy and metabolism during fasting
The coming of spring harkens spring cleaning; a time to de-clutter your home and discard things that are no longer needed. In the body, a cellular process called autophagy occurs regularly to “de-clutter” our cells. Recently, researchers in Japan have shed new light on the relationship between this process and the body’s metabolic response to fasting.
In a new study published in Autophagy, researchers led by Osaka University investigated the role of autophagy. Autophagy is the process by which unwanted cellular components are eliminated via degradation, during fasting conditions.
Previous studies have shown that fasting causes fat tissue, also known as adipose tissue, to break down, which leads to hepatic steatosis (an accumulation of fat in the liver) and ketogenesis (the production of ketones, which are by-products of fat breakdown). A gene called Rubicon Autophagy Regulator (RUBCN) acts as a negative regulator of autophagy, meaning that it functions to suppress autophagy. The research team led by Osaka University previously demonstrated that the loss of RUBCN in fat tissue during aging leads to systemic fat loss. Because RUBCN levels are also reduced during fasting, the researchers hypothesized that this reduction may promote fat loss through the upregulation of autophagy.
“We wanted to further our understanding of how autophagy is involved in the body’s metabolic response to fasting,” says lead author Tadashi Yamamuro. “To do so, we evaluated the effects of modulating autophagy in the adipose cells of fed and fasting mice.”
The researchers used several mouse models to perform their investigation, including an adipose-specific model that lacked RUBCN and an adipose-specific model that lacked ATG5, a gene that is essential for autophagy to occur. Fat loss, triglyceride levels, and liver weight were evaluated in these mice and compared with control mice under fed and fasting conditions.
“In control mice, fat loss, hepatic steatosis, and ketonemia were observed under fasting conditions,” says senior author Tamotsu Yoshimori. “Fed RUBCN knockout mice exhibited responses that were similar to those of fasted control mice, while fasted ATG5 mice exhibited reduced fat loss, hepatic steatosis, and ketonemia.”
The researchers also found that fasted control mice exhibited a substantial decrease in the expression of adipogenic, or fat-promoting, genes. In ATG5 knockout mice, this reduction was not observed, indicating that autophagy plays a role in the reduction of adipogenic gene expression.
Taken together with the findings from the research team’s previous study, it appears that upregulation of autophagy in adipose tissue is a hallmark of both fasting and aging. In addition to revealing a previously unknown mechanism of the fasting response, these findings may have important implications for our understanding of metabolism during aging.
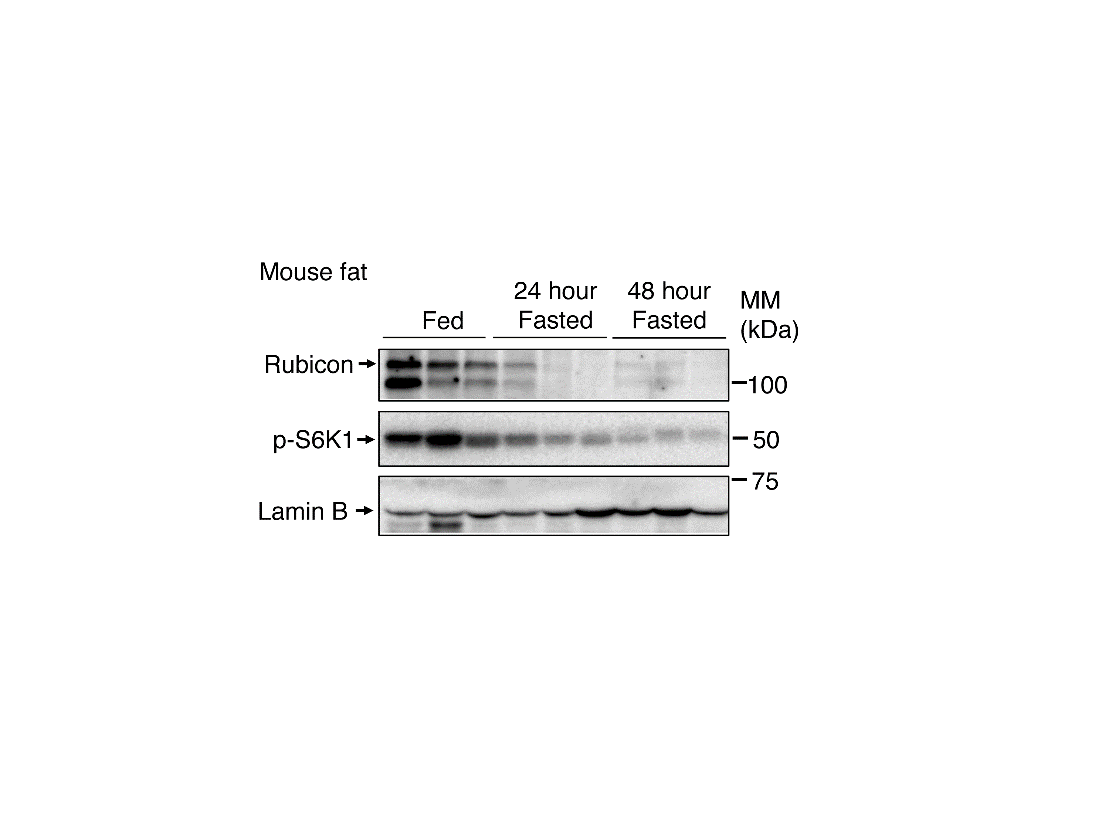
Fig.1 Rubicon in mouse adipose tissue decreases during fasting.
(Mouse adipose tissue) The panel shows the protein amount in adipose tissue. Fasted mice exhibited a significant reduction in Rubicon in adipose tissue compared with fed mice. mTORC1 activity indicator p-S6K1 was also reduced in fasting conditions, which suggests that mTORC1 is inactivated with fasting. Lamin B was used as the loading control.
(credit: Tadashi Yamamuro et al.)
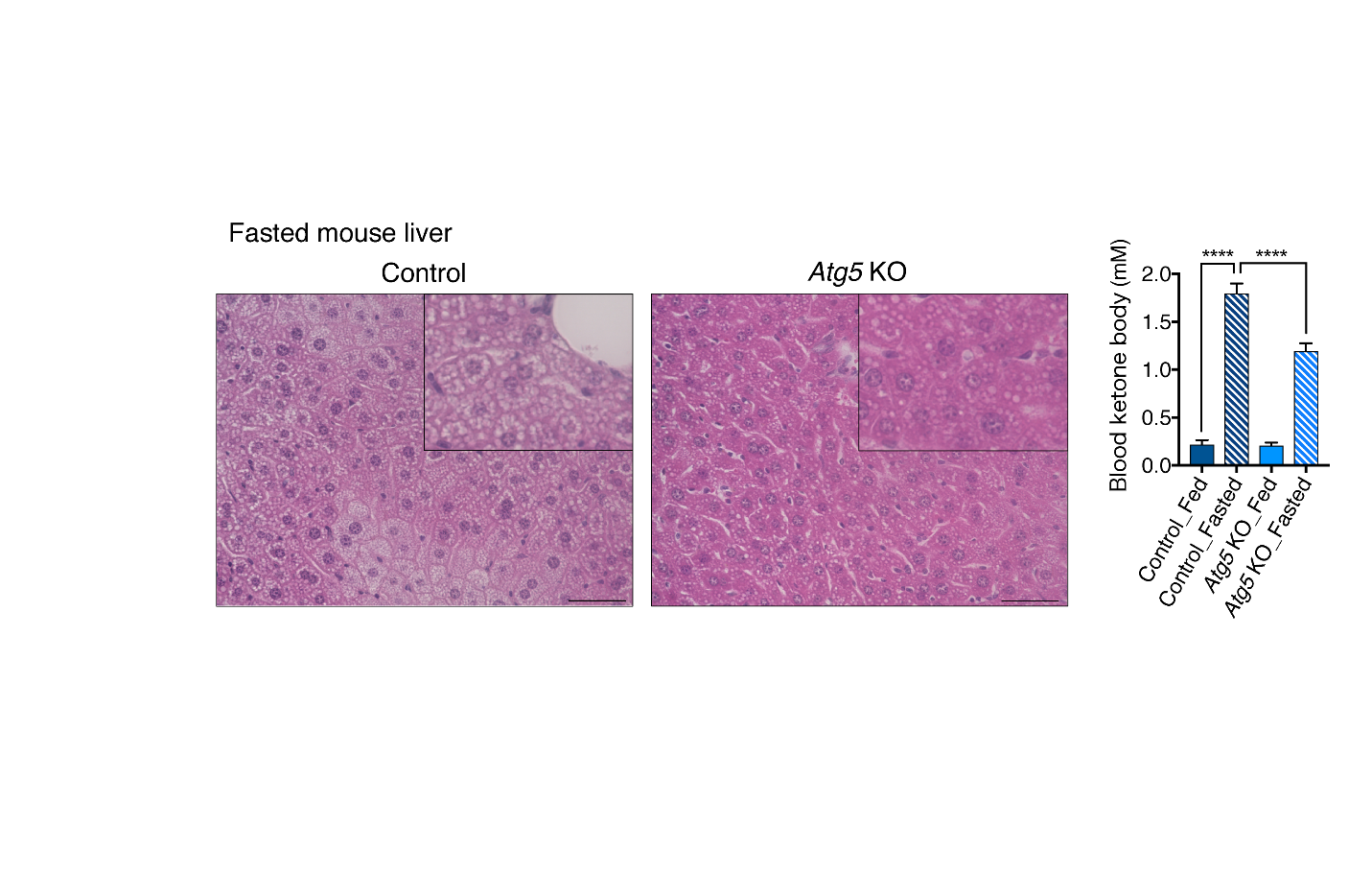
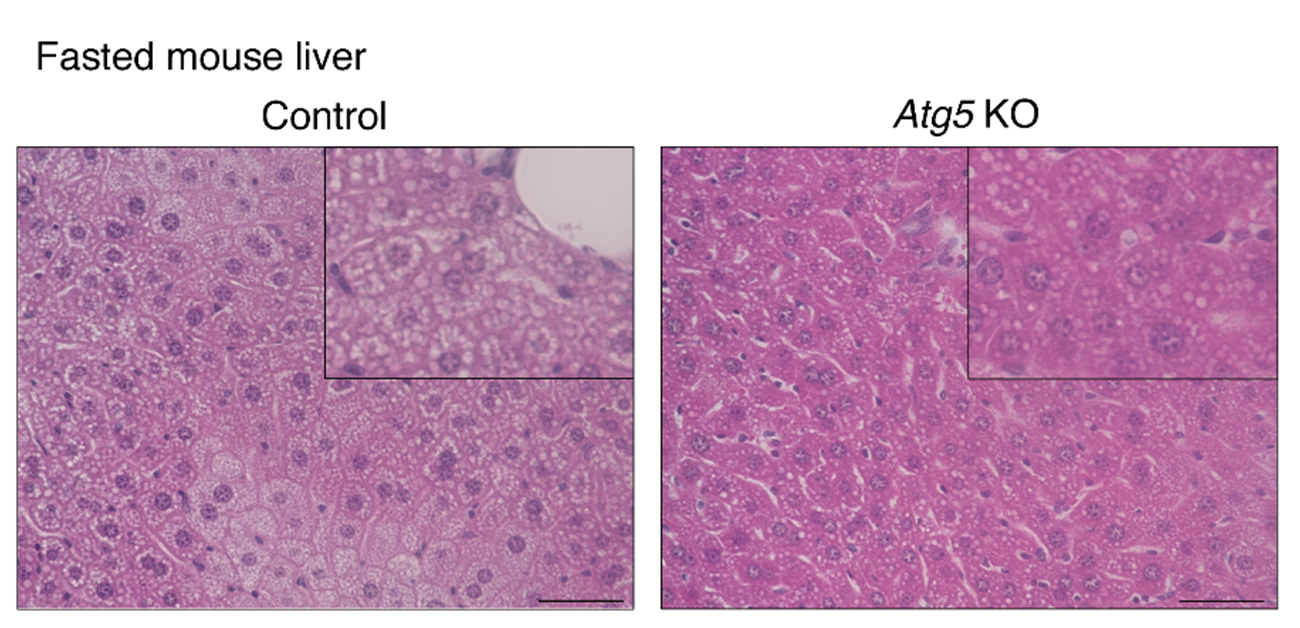
Fig.2 Suppression of autophagy in adipose tissue decreases hepatic steatosis and ketogenesis during fasting.
(Left: Mouse liver) The panel shows the Hematoxylin-Eosin staining in the liver from 24-h-fasted mice. Control mice exhibited a significant accumulation of lipid droplets, whereas this accumulation was reduced in adipose-specific Atg5 knockout mice in which autophagy was suppressed.
(Right: Blood ketone body level) The panel shows the blood ketone body levels in fed or 24-h-fasted mice. Control mice exhibited a fasting-induced increase in blood ketone body levels, whereas this increase was impaired in adipose-specific Atg5 knockout mice in which autophagy was suppressed. This result indicates that adipose autophagy is required for ketone body production in the liver.
(credit: Tadashi Yamamuro et al.)
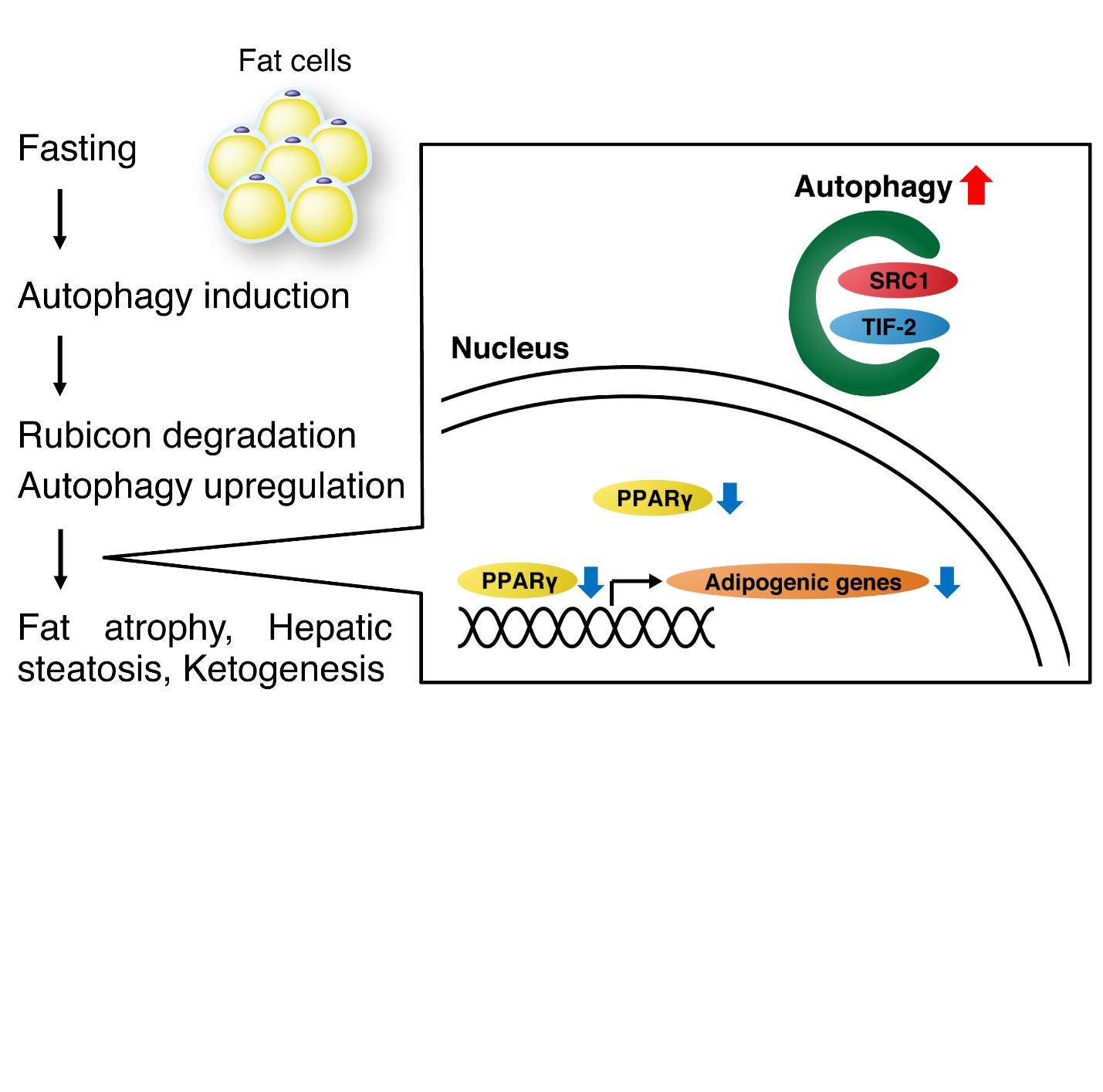
Fig.3 The mechanism of the fasting response in adipocytes
Adipose autophagy is induced with fasting, thereby degrading Rubicon to further promote autophagy. Autophagy in adipocytes degrades SRC-1 and TIF2, and their reduction leads to a decrease in adipogenic gene expression and fat mass. Fat is transferred from adipose tissue to the liver and used for ketone body production.
(credit: Tadashi Yamamuro et al.)
The article, “Loss of RUBCN/rubicon in adipocytes mediates the upregulation of autophagy to promote the fasting response,” was published in Autophagy at DOI: https://doi.org/10.1080/15548627.2022.2047341.
Related Links
Yoshimori Tamotsu (Researcher Directory)
