Link discovered between male infertility and self-eating
Researchers from Osaka University find that excessive autophagy, or programmed cell death, may be linked to male infertility
Androgens—male hormones—are involved in male fertility. However, it has previously been elusive how androgens affect the creation of sperm. Now, a team from Osaka University has found that androgens regulate the levels of a protein called Rubicon to control the breakdown of another protein called GATA4 that is essential for the proper functioning of Sertoli cells.
Androgens become downregulated upon fatherhood, with an associated decrease in fertility, which may occur to focus a father towards caring for his existing offspring. Sertoli cells are a cell type essential for spermatogenesis, or the generation of sperm, that are located in the testes. Until now, it has been unclear how the functions of Sertoli cells are regulated, or how they are affected by androgens.
Rubicon negatively regulates autophagy, so low levels of Rubicon lead to increased autophagy. These low levels and the resultant increased autophagy in the testis led to decreased spermatogenesis. “Rubicon is therefore likely to be responsible for inhibiting the degradation of GATA4 and protecting Sertoli cell function,” explains lead author Tadashi Yamamuro. “Then, Rubicon itself may be regulated by androgens, that act to maintain the levels of Rubicon and GATA4 in cells.” Therefore, it seems that Rubicon’s effect on autophagy may control the amount of GATA4 in various organs during development.
Interestingly, while genetic loss of Rubicon decreases male fertility, Rubicon is known to accumulate with age, and its loss extends lifespan by upregulating autophagy. Increased autophagy is beneficial in terms of age-related diseases and is associated with longevity. “Fertility is known to be negatively correlated with longevity in animals,” says senior author Shuhei Nakamura, “and this regulation of autophagic GATA4 degradation by Rubicon could be one of the underlying mechanisms that reciprocally regulates both fertility and longevity.”
As well as the discovery of this novel mechanism by which Sertoli cells are regulated, some male infertility conditions could well be a result of excess autophagy in Sertoli cells. These findings may therefore aid in the development of treatments for male infertility.
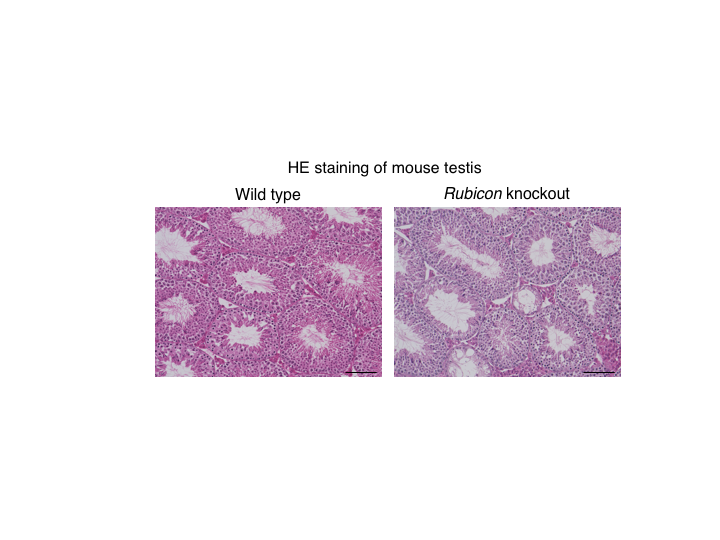
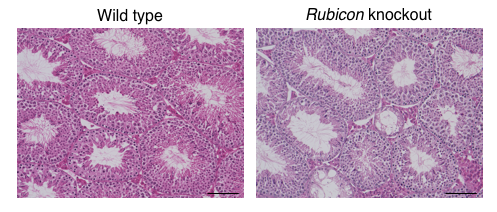
Figure 1. Systemic Rubicon-knockout mice exhibit a reduction in spermatogenesis.
Hematoxylin-Eosin staining of mouse testes. Compared with wild-type mice, systemic Rubicon-knockout mice show a decrease in spermatogenesis. (credit: Tadashi Yamamuro et al., No change made. https://creativecommons.org/licenses/by/4.0/)
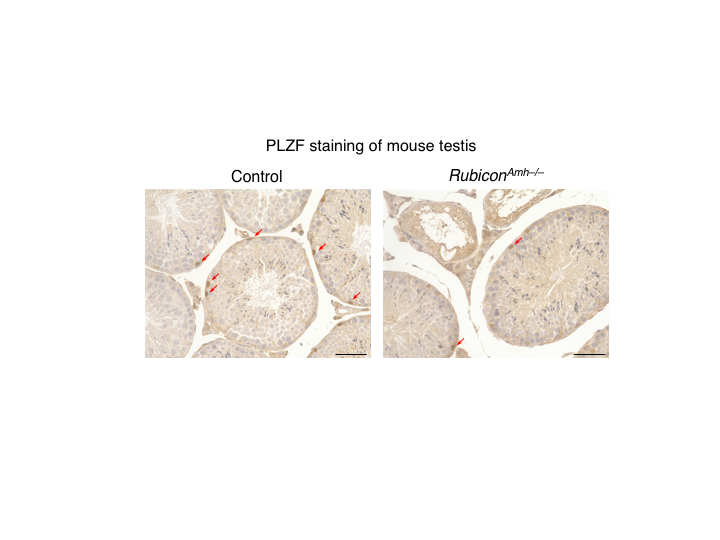
Figure 2. RubiconAmh–/– mice exhibit a decrease in the number of spermatogonial stem cells.
PLZF staining of mouse testes. PLZF is a marker for spermatogonial stem cells. Compared with control mice, Sertoli-cell-specific Rubicon knockout mice (RubiconAmh–/– mice) show a reduction in the number of spermatogonial stem cells. (credit: Tadashi Yamamuro et al., No change made. https://creativecommons.org/licenses/by/4.0/)

Figure 3. Regulation mechanism of GATA4 in Sertoli cells.
Rubicon negatively regulates autophagic degradation of GATA4 in Sertoli cells. Upon anti-androgen therapy, the levels of Rubicon in Sertoli cells decrease to promote autophagic degradation of GATA4.
The article, “Rubicon prevents autophagic degradation of GATA4 to promote Sertoli cell function,” was published in PLOS Genetics at https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1009688.
