Unidentified interaction sites of immunoglobulin G1 antibodies and their receptors discovered
A group of researchers from Nagoya City University and Osaka University conducted joint research on antibody molecules and their receptors in the immune system, identifying interaction sites outside of previously identified receptor-interaction sites.
The immune system protects our body from harmful foreign substances. When we get invaded by foreign substances (called antigens), the body responds with the immune system in order to remove these antigens from the body using antibodies. Antibodies, proteins present in the blood, specifically recognize the antigens, such as bacteria, viruses, and cancer cells, and activate the immune system to get rid of them by binding to immune cells.
By taking advantage of the property of antibodies that allows them to only bind to a specific antigen, many biotechnologically manufactured drugs are used. Biotechnologically manufactured medicines for treating cancer use immune response via interactions between antibodies and immune cells. The strength of antigen-antibody interaction, the antibody’s affinity for the antigen, is determined by the strength of the interaction between the antibody and a single binding site, so the avidity has been drawing attention. Fc receptors on the surface of immune cells bind to the Fc portion of antibodies in order to play a role in modulating immune cell activity.
The Fab portion of the antibody binds to the antigen, whereas the Fc portion binds to an Fc receptor on immune cells. It was widely assumed that the interaction of FcγR with IgG was mediated through the Fc region, and previous studies demonstrated their FcγR interactions with IgGs were inhibited by the Fc fragments but not the Fab fragment.
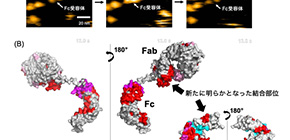
In this study, using high-speed atomic force microscopy (HS-AFM) and human, humanized, and mouse/human-chimeric IgG1 antibodies and their Fc fragments, along with their cognate Fc receptor, FcγRIIIa, the group of researchers performed real-time observation of IgG-FcγR interactions.
The HS-AFM data showed that the dwell times of IgG1 molecules on clustering FcγRIIIa were significantly longer than those of their Fc fragments, indicating that their Fab portions stabilized the complexes formed with the receptor. The group also identified the Fc receptor’s binding sites in the Fab region of IgG1 by Hydrogen/Deuterium eXchange Mass Spectrometry (HDX-MS) analysis.
In this study, the researchers performed real-time observation of IgG-FcγR interactions which had been overlooked because of the difficulty of analysis, finding that both Fc and Fab portions are involved in the interaction with Fc receptor FcγRIIIa. By changing receptor-interaction sites in IgG-Fab clarified in this study, antibody drugs with higher therapeutic efficacy will be developed.
Figure 1
The article “The Fab portion of immunoglobulin G contributes to its binding to Fcγ receptor III” was published in Scientific Reports at DOI: https://doi.org/ 10.1038/s41598-019-48323-w .
Related links
