Intracellular information is diffused through assembly and dissociation of Wnt protein complexes by regulating information diffusion range
Tissues and organs in animals are made of many cells and exhibit distinct shapes and functions. Intercellular communications are essential for forming these tissues and organs and for maintaining their homeostasis. Wnt proteins, molecules involved in intercellular signaling, are secreted from specific cells in tissues and accepted by surrounding cells to mediate intercellular communications. It is important to know the Wnt diffusion range in order to understand characteristics of intercellular communications in tissues and organs, but how the range was determined was unknown.
A group of researchers at the Exploratory Research Center on Life and Living Systems (ExCELLS), National Institute for Basic Biology, together with the groups at Osaka University, National Institute of Advanced Industrial Science and Technology (AIST), and RIKEN, clarified that Wnt proteins secreted into the extracellular milieu formed complexes that had trimers as basic units, proposing a new idea that the Wnt diffusion range was determined by a balance between the assembly of Wnt complexes and their dissociation.
Wnt has drawn attention as a signaling protein that plays an important role in various biological phenomena, such as the generation of animals, differentiation of cells, stem cell homeostasis, and cancerization, but the mechanisms of their higher-order structures and diffusion were unclear.
Using analytical ultracentrifugation with a fluorescence detection system and single-particle analyses by gel filtration chromatography, the researchers found:
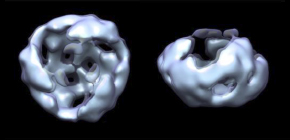
- 1. Wnt proteins secreted into the extracellular milieu formed complexes the homo-trimer.
- 2. This homo-trimer further assembled with each other into higher-order structures and became dissociable by interaction with the extracellular domain of the receptor or secreted Wnt-binding protein.
In addition, they also found that the assembly of Wnt complexes and their dissociation happened in embryos as well. Large high-molecular-weight (HMW) complexes were less mobile, but when they were dissociated by interaction with soluble Wnt-binding protein, the diffusion range of Wnt proteins expanded.
This study revealed the basic mechanism for determining the Wnt diffusion range. This is an important discovery in clarifying molecular mechanisms of intercellular communications, which are a foundation of genesis and homeostasis of tissues and organs.
Abstract
Members of the Wnt protein family play roles in many aspects of embryogenesis and homeostasis. Despite their biological significance, characteristics of Wnt proteins still remain unclear, mainly due to their insolubility after the removal of serum. Here we examine Wnt proteins in serum-containing media by using analytical ultracentrifugation with a fluorescence detection system. This analysis reveals that Wnt3a assembles into high-molecular-weight complexes that become dissociable by interaction with the extracellular domain of the Frizzled8 receptor or secreted Wnt-binding protein sFRP2. Cross-linking and single-particle analyses of Wnt3a fractionated by gel filtration chromatography show the homo-trimer to be the smallest form of the assembled Wnt3a complexes. Fluorescence correlation spectroscopy and immunohistochemistry reveal that the assembly of Wnt3a complexes restricted their diffusion and signaling range in Xenopus laevis embryos. Thus, we propose that the Wnt diffusion range can be controlled by a balance between the assembly of Wnt complexes and their dissociation.
Figure 1
Figure 2
Figure 3
The article, "Assembly of protein complexes restricts diffusion of Wnt3a proteins," was published in Communications Biology at DOI: https://doi.org/10.1038/s42003-018-0172-x
Related links
