Elucidation of the Mechanism Underlying the Initiation of the Clearance of TDP-43
May contribute to establishment of treatment methods for nervous diseases such as ALS
A group of researchers led by Professor Yukio KAWAHARA , Department of RNA Biology and Neuroscience, Graduate School of Medicine, clarified the biological significance of the fragmentation of TDP-43, a RNA-binding protein associated with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). They found that endoplasmic reticulum (ER) membrane-bound caspase-4 monitors the amount of TDP-43 in the ER and when the ER is overloaded with TDP-43, caspase-4 cleaves a small portion of TDP-43. This cleavage triggers the downstream caspase-3/7 pathway to promote fragmentation to reduce full-length TDP-43-mediated cytotoxicity. Therefore, increasing caspase-4 activity or the rate of TDP-43 clearance might have therapeutic potential for ALS and FTLD.
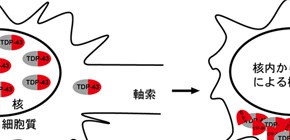
TDP-43 is a 43kDa protein involved in multiple aspects of RNA metabolism. It is localized predominantly in the nucleus in healthy neurons ( Fig.1 ). However, it is cleared out from the nucleus and accumulated in the cytoplasmic aggregates in the degenerative neurons in the patients with ALS, a motor neuron disease, and FTLD, a dementia disease. Furthermore, TDP-43 protein is abnormally phosphorylated, ubiquitinated, and truncated in degenerative neurons. This results in the accumulation of an approximately 25-kDa C-terminal fragment (CTF25) in cytoplasmic aggregates ( Fig.1 ). Consequently, both loss of nuclear function of TDP-43 and gain of toxic function due to the formation of the aggregates have been proposed as causative mechanisms underlying neuronal death.
We can observe the production of CTF25 by the overexpression of TDP-43 in cultured cells and animals. This suggests that TDP-43 is degraded through a mechanism that involves the generation of CTF25. However, the cleavage site that generates CTF25 and biological significance of the cleavage remain undetermined. This group identified Asp174 as a predominant cleavage site for CTF25 ( Fig.2 ). TDP-43 is cleaved initially after Asp174, which activates caspase-3/7 to accelerate TDP-43 fragmentation ( Fig.3 ). Consequently, blockage of this cleavage results in a severe delay in TDP-43 clearance and prolonged necrotic cell death. They further show that endoplasmic reticulum (ER) membrane-bound caspase-4 is the enzyme responsible for the cleavage after Asp174 and inhibition of caspase-4 activity slows TDP-43 fragmentation and reduces cell viability ( Fig.3 ). These findings suggest that caspase-4-mediated cleavage after Asp174 is an initiator of TDP-43 clearance, which is required to avoid cell death induced by overexpressed TDP-43. Therefore, it is expected that the molecules that can increase caspase-4 activity or the rate of TDP-43 clearance might prevent the progression of ALS and FTLD.
To learn more about this research, please view the full research report entitled " The cleavage pattern of TDP-43 determines its rate of clearance and cytotoxicity " at this page of Nature Communications website.
Related link
