Role of Ataxin-2 in development of ALS identified
Contributions to pathogenesis and treatments expected
A group of researchers led by KAWAHARA Yukio (Professor, Gene Therapy Science, Department of Genome Biology, Graduate School of Medicine, Osaka University) and SUZUKI Yutaka (Professor, Graduate School of Frontier Sciences, The University of Tokyo) have clarified the function of Ataxin-2 protein, a key player in the development of neurodegenerative diseases such as Amyotrophic Lateral Aclerosis (ALS), SpinoCerebellar Ataxia type 2 (SCA2), Parkinson's disease. Ataxin-2 has been known to be related to the development of neurodegenerative diseases; however, specifics in the functioning of Ataxin-2 have been unknown.
By making use of the PAR-CLIP (Photoactivatable-Ribonucleoside-Enhanced Crosslinking and Immunoprecipitation) and analysis by next-generation sequencing, this group succeeded in identifying the binding sites of RNA-binding proteins, Ataxin-2s. As a result, this group found that Ataxin-2 promotes RNA stability and that genetic mutations in ALS and SCA2 lowered Ataxin-2's capability to stabilize RNA.
This group's results are expected to contribute to the clarification of the pathogenesis of neurodegenerative diseases and the establishment of new methods for treating such.
Abstract
It has been proposed that Ataxin-2, a member of the like-Sm (LSm) protein family, participates in the regulation of RNA metabolism through interaction with PABPC1. However, the exact biological mechanism and in vivo targets remain unknown. Here, we report that Ataxin-2 binds directly to RNAs in a PABPC1-independent manner. High-throughput sequencing of Ataxin-2-bound RNAs prepared by PAR-CLIP revealed that Ataxin-2 binds predominantly to uridine-rich elements, including well-characterized cis-regulatory AU-rich elements, in the 3′ UTRs of target mRNAs. Gene expression analysis after Ataxin-2 depletion or overexpression revealed that Ataxin-2 stabilizes target mRNAs and increases the abundance of the corresponding proteins. A tethering assay demonstrated that Ataxin-2 elicits this effect by direct interaction with mRNAs. We also found that disease-associated polyglutamine expansion downregulates the physiological activity of Ataxin-2. These findings suggest that Ataxin-2 is an RNA-binding protein that targets cis-regulatory elements in 3′ UTRs to stabilize a subset of mRNAs and increase protein expression.
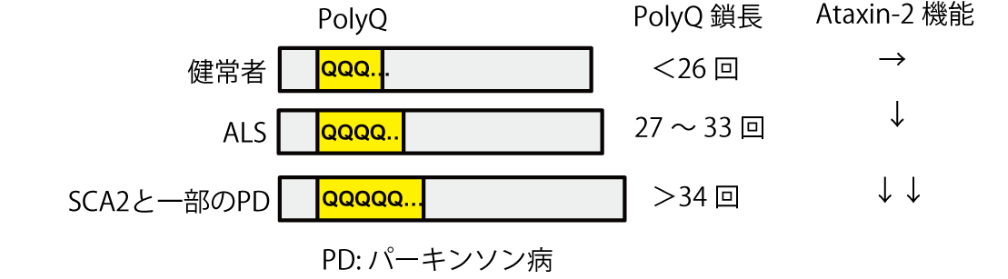
Figure 1
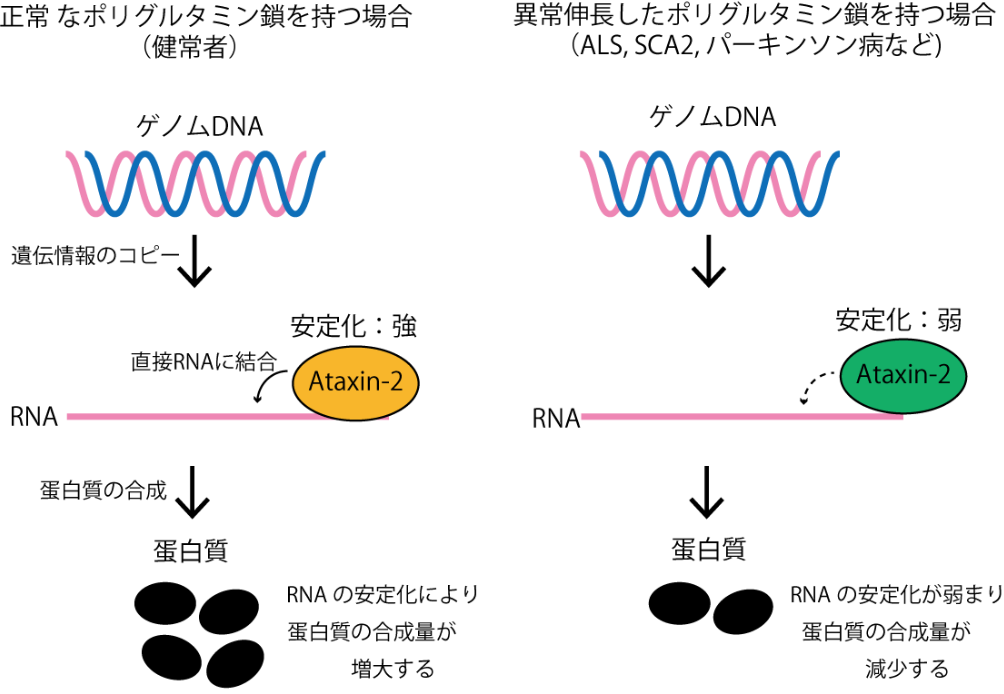
Figure 2
To learn more about this research, please view the full research report entitled " Direct Binding of Ataxin-2 to Distinct Elements in 3′UTRs Promotes mRNA Stability and Protein Expression " at this page of the Molecular Cell website.
Related link :
