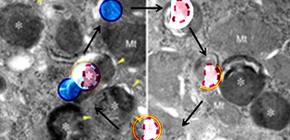
Discovery of mechanism for sequestering and recovering damaged lysosomes
A group of researchers led by YOSHIMORI Tamotsu (Professor, Graduate School of Frontier Biosciences, Graduate School of Medicine, Osaka University) and MAEJIMA Ikuko (CREST Researcher) and a group led by ISAKA Yoshitaka (Associate Professor, Graduate School of Medicine, Osaka University) clarified that damaged lysosomes were selectively sequestered and recovered by autophagy. When lysosomes, subcellular organelles, rupture, digestive enzyme and active oxygen are released making the damaged lysosomes hazardous to the cell.
This joint group discovered that autophagy cleaning up waste in cells detected damaged lysosomes and sequestered them effectively. The number of lysosome per cell is stable regardless of whether the cell is damaged or not. If the damaged lysosomes are not removed, new lysosome will not be made and the cell's digestion capacity will decline. In experiment using mice, this joint group has found that nephropathy caused by hyperuricemia deteriorated if autophagy did not sequester the lysosomes damaged by urate crystals.
Recently it has been found that some factors of lifestyle-related diseases such as type 2 diabetes, arterial sclerosis, and gout damaged lysosomes and these diseases decreased autophagy. Thus, the development of new medical treatments involving autophagy is expected.
Abstract
Diverse causes, including pathogenic invasion or the uptake of mineral crystals such as silica and monosodium urate (MSU), threaten cells with lysosomal rupture, which can lead to oxidative stress, inflammation, and apoptosis or necrosis. Here, we demonstrate that lysosomes are selectively sequestered by autophagy, when damaged by MSU, silica, or the lysosomotropic reagent L-Leucyl-L-leucine methyl ester (LLOMe). Autophagic machinery is recruited only on damaged lysosomes, which are then engulfed by autophagosomes. In an autophagy-dependent manner, low pH and degradation capacity of damaged lysosomes are recovered. Under conditions of lysosomal damage, loss of autophagy causes inhibition of lysosomal biogenesis in vitro and deterioration of acute kidney injury in vivo. Thus, we propose that sequestration of damaged lysosomes by autophagy is indispensable for cellular and tissue homeostasis.

Figure 1

Figure 2
To learn more about this research, please read the full research report entitled " Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury " at this page of the EMBO Journal website.
Related links :
