Mechanism behind fatty liver elucidated
Autophagy suppression factor Rubicon causes lipid accumulation in the liver and hepatic disorder
Fatty liver (lipid accumulation in hepatic cells) caused by hypernutrition is on the rise in Japan and other developed countries. It is said that about 30 percent of population develop fatty liver, making it a very common lifestyle-related disorder. Some people with fatty liver disease develop serious nonalcoholic steatohepatitis (NASH), which develops into serious diseases such as liver cirrhosis and hepatic cancer, so it’s a challenge to suppress exacerbation of fatty liver. However, there are no effective medicines for treating fatty liver, so it is hoped that a more complete understanding of the condition of the disease will lead to the development of its treatment methods.
A group of researchers led by Graduate Student TANAKA Satoshi, Assistant Professor HIKITA Hayato, Professor TAKEHARA Tetsuo, and Professor YOSHIMORI Tamotsu at the Graduate School of Medicine, Osaka University, clarified that increased expression of Rubicon in the liver caused fatty liver.
It was reported that autophagy, a destructive mechanism of the cell, was suppressed in fatty liver, but its details were unknown. This group found that expression of Rubicon increased and autophagy was suppressed in cultured human liver fat-storing cells (HepG2, a human liver carcinoma cell line) and in mouse liver cells that were fed a high-fat diet (32% fat, 4 months).
This group also clarified that suppression of expression of Rubicon reduced lipid accumulation and cellular death in the mouse liver and that the exacerbation of fatty liver was caused by compromised autophagy due to increased expression of Rubicon. This group confirmed increased expression of Rubicon in the liver of NASH patients as well.
This group’s achievement will lead to the development of drugs for reducing patients’ hepatic fat and relieving their hepatic disorder by targeting Rubicon. It is also hoped that this will delay the onset of NASH and hepatic cancer.
It was known that some congenital disorders (genetic disorders) developed due to autophagy compromised by mutation, but this group demonstrated an acquired environment, the intake of high-fat diet in this study, also compromised autophagy, developing disorders, a world first. This group’s achievement also draws attention in terms of hypernutrition, an urgent issue in modern society.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver disease worldwide. It encompasses a spectrum ranging from simple steatosis to fatty liver with hepatocellular injury, termed nonalcoholic steatohepatitis. Recent studies have demonstrated hepatic autophagy being impaired in NAFLD.
In the present study, we investigated the impact of Rubicon, a Beclin1-interacting negative regulator for autophagosome-lysosome fusion, in the pathogenesis of NAFLD. In HepG2 cells, BNL-CL2 cells and murine primary hepatocytes, Rubicon was post-transcriptionally upregulated by supplementation with saturated fatty acid palmitate. The upregulation of Rubicon was associated with suppression of the late stage of autophagy as evidenced by accumulation of both LC3-II and p62 expression levels as well as decreased autophagy flux. Its blockade by siRNA attenuated autophagy impairment and reduced palmitate-induced endoplasmic reticulum stress, apoptosis and lipid accumulation. Rubicon was also upregulated in association with autophagy impairment in the livers of mice fed a high-fat diet. Hepatocyte-specific Rubicon knockout mice generated by crossing Rubicon floxed mice with albumin-Cre transgenic mice did not produce any phenotypes on a normal-diet. In contrast, on a high-fat diet, they displayed significant improvement of both liver steatosis and injury as well as attenuation of both ER stress and autophagy impairment in the liver. In human, liver tissues obtained from patients with non-alcoholic fatty liver disease expressed significantly higher levels of Rubicon than those without steatosis.
Conclusion: Rubicon is overexpressed and plays a pathogenic role in NAFLD by accelerating hepatocellular lipoapoptosis and lipid accumulation, as well as inhibiting autophagy. Rubicon may be a novel therapeutic target for regulating NAFLD development and progression.

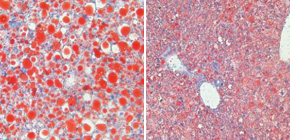
Fig 1. Pathogenic mechanism of fatty liver disease
High-fat diet increases Rubicon expression in hepatocytes, which suppress lipid degradation and induce apotosis.
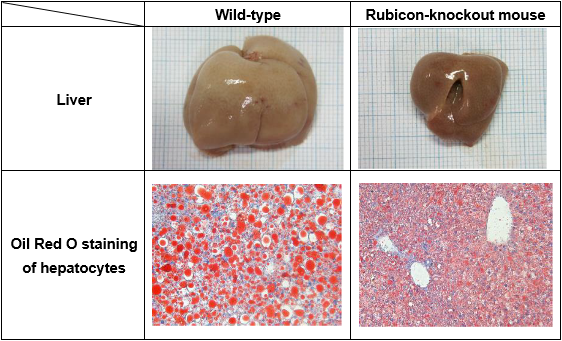
Fig 2 . Comparison of liver and hepatocytes between normal mice and hepatocyte-specific Rubicon-knockout mice on a high-fat diet.
Hepatocyte-specific knockout of Rubicon reduces lipid accumulation (shown in red) and bloated liver size in mice on a high-fat diet.
To learn more about this research, please view the full research report entitled “ Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease ” at this page of the Hepatology website.
Related links
