
Clarification of innate immune cell mechanism in the suppression of intestinal inflammation in mice
Synopsis
Under the leadership of Professor TAKEDA Kiyoshi and Assistant Professor KAYAMA Hisako of the Graduate School of Medicine, Osaka University , a team of researchers has succeeded in clarifying a mechanism occurring in certain innate immune cells*1 in the intestinal mucous membrane and the fact that abnormalities in such innate immune cells are causally related to the development of inflammatory bowel diseases (IBC)*2.
Abnormalities in adaptive immune cells have been thought to be the main cause of inflammation in the development of IBD, so conventional research has focused on the adaptive immune cells*3. It has been known that innate immune cells mediate adaptive immune cell activity; however, the extent of the role of innate immune cells in the development IBD has been unclear.
This group has clarified that certain innate immune cells bind with adaptive immune cells, suppressing their activity and named these innate immune cells " regulatory myeloid cells " (Mreg). The group also clarified that normal Mreg cells given to model mice with IBD reduced the level of inflammation in the bowels of the mice. This research result clarified the potential of innate immune cells to suppress bowel inflammation and will lead to the development of new medical treatments for IBD.
This research project was conducted in cooperation with MURAKAMI Masaaki, Associate Professor at the Graduate School of Frontier Biosciences, ISHII Masaru, Professor at the Immunology Frontier Research Center, OKAZAKI Taku, Professor at the Institute for Genome Research, The University of Tokushima, and YAGITA Hideo, Full-time Associate Professor at the School of Medicine, Juntendo University.
The research result was published in the online breaking news of the Proceedings of the National Academy of Sciences ( PNAS ) of the United States of America in the week of March 5, 2012 (US Eastern Time).
Background
Immune cells*4 play an important role in our immune system by attacking and removing foreign bodies such as bacteria and viruses.
Innate immune cells activate adaptive immunity by engulfing pathogens and alerting adaptive immune cells, T cells, of the pathogen's antigens. However, because antigens from foreign substances such as the 10 trillion intestinal bacteria and foods consumed are always present in the intestines, excessive immune cell response to such substances in the intestinal mucosa break down intestinal tissues, leading to the development of bowel inflammation.
Therefore, immune cells in the intestinal mucosa need to have a certain level of immune tolerance*5, a condition in which the immune cells do not attack foreign bodies. It has been reported that a decrease in immune tolerance in the intestinal tissues may be a cause of IBC, a condition that has been drastically increasing in Japan. Heretofore the causative agent and pathogenic mechanism in IBD had been unknown and a method for greatly improving the clinical situation had not been established.
As IBD features an abnormal increase in T cells inducing inflammation, conventional approaches for clarifying the cause and pathogenic mechanism of IBD have focused on the adaptive immune system and the T cells which play a major role. However, it has been clarified recently that abnormalities in innate immune cells interfere with suppression of the adaptive immune system. Thus, the role of innate immune cells in the maintenance of homeostasis in the intestinal tissues is receiving closer attention.
Adaptive immune cells known as Regulatory T cells (Tregs) play an important role in the maintenance of immune tolerance in the intestines and the suppression of intestinal inflammation because Tregs suppress activation of inflammatory T cells. While some instances of innate immune cells in the intestinal mucosa indirectly suppressing inflammation by inducing Treg cell activity have been reported, no innate immune cell activity directly suppressing inflammatory T cells has been reported. For this reason, this group decided to examine whether innate immune cells, without mediation from Tregs, were able to directly suppress bowel inflammation.
Findings
In order to find innate immune cells capable of inhibiting T cell proliferation, this group focused on the largest cell population in the innate immune cells taken from the mouse intestinal mucosa. The group found three subsets in these cells. (Figure 1A) One of the cell subsets was found to suppress T cell proliferation. (Figure 1B) Further investigation clarified that such cells generate many cell adhesion molecules*6 and easily bind with inflammatory T cells.
Next, in order to analyze the function of these cells in organisms, these cells were administered to the abdominal cavities of IBD model mice. These cells were found to suppress bowel inflammation by preventing inflammatory T cells from proliferating in the intestinal tissues. (Figure 1C)
These cells do not induce Tregs but suppress proliferation by directly combining with inflammatory T cells through their cell adhesion molecules. Based on this evidence, the group named these new cells regulatory myeloid cells, Mregs.
In order to further clarify the molecular mechanism for suppression of bowel inflammation by Mreg cells, this group conducted a gene expression analysis. They found that in Mreg cells there are many genes whose expression is induced by anti-inflammatory cytokine IL-10 and Stat3 transcription factor*7, found in large amounts in the intestinal mucosa.
Thus, this group theorized that IL-10 and Stat3 were needed to suppress T cells from proliferation by Mreg cells.
In order to verify this hypothesis, the group cultivated Mreg cells and inflammatory T cells with damaged IL-10 and/or Stat3. The result was the proliferation of inflammatory cells was not suppressed. Furthermore, this group administered Mreg cells with damaged Stat3 into the mouse abdominal cavities, but again bowel inflammation was not suppressed. Based on this, they concluded that Mreg cells need IL-10 or Stat3 to suppress proliferation of inflammatory T cells and bowel inflammation. (Figure 2A)
Further analysis clarified that, in Mreg cells, the expression of proteins such as CD80 and CD86 essential for the activation of T cells was suppressed by IL-10 and Stat3. When normal Mreg cells were injected into the bowel cavities of IBD model mice, the bowel inflammation decreased. (Figure 2B) These results clarified that IL-10 or Stat3 in Mreg cells played an important role in prevention of intestinal inflammation.
From these research results, they concluded the following two steps in the suppression of inflammatory T cells and the maintenance of homeostasis in the intestinal immune system:
1. Mreg cells in the intestines generate significant quantities of adhesion molecules and combine with inflammatory T cells more readily than other types of T cells.
2. IL-10 and/or Stat3 in Mregs inhibit the generation of CD80 and CD86 necessary for the activation of inflammatory T cells, preventing proliferation of inflammatory T cells.
Future development
This research clarified that Mreg cells, one type of innate immune cells in mouse intestines, suppress the development of intestinal inflammation and that the injection of Mreg cells to the abdominal cavities of mice with intestinal inflammation has treatment efficacy. The identification of human Mreg cells and clarification of their action mechanism will lead to the development of effective IBD treatment methods.
Recently it has been reported that the breakdown of immune tolerance in the intestines is associated with changes in gut flora*8 and is deeply involved in the development of autoimmune diseases such as IBD and multiple sclerosis. These intractable diseases are increasing in Japan in step with changes in life style and Western-style diet.
Identification of Mreg cells in humans coupled with methods for increasing the number of Mreg cells will make possible the development of therapeutic methods for IBD and many other autoimmune diseases.

Figure 1
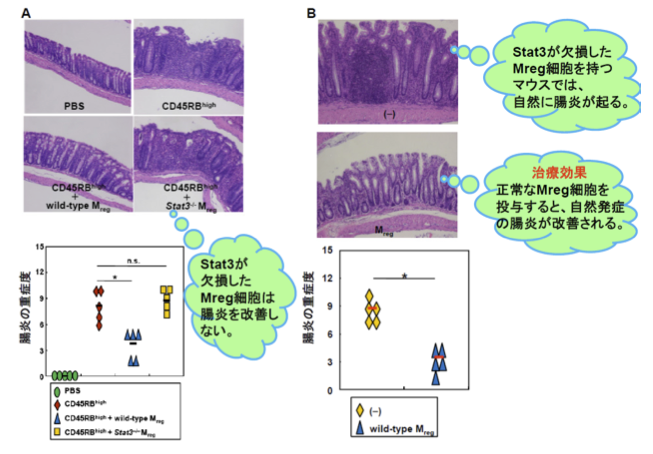
Figure 2
Notes :
1. Innate immune cells -- Immune cells function as a first phase response to a pathogen when it enters the body. Capable of recognizing foreign substances, immune cells activate themselves and engulf the pathogen. Macrophage and dendritic cells are included in this type of cell.
2. Inflammatory bowel diseases (IBC) -- An autoimmune disease. Immune cells attack the intestinal mucosa, causing inflammation. The cause of the development of IBC has not been clarified. IBC includes ulcerative colitis and Crohn's disease.
3. Adaptive immune cells -- are highly specialized, systemic cells and processes that eliminate or prevent pathogenic growth. T cells produce antibodies (B cells) against specific pathogens that the innate immune cells have engulfed, triggering adaptive immune response against the pathogens. Key cells are T cells and B cells.
4. Immune cells -- Cells that work at identifying and eliminating pathogens and cancer cells in the body. The immune cell system is divided into two major branches: the innate immune system and the adaptive immune system. We are born with our innate immune system. The adaptive immune system reacts against specific antigens. Immune cells originally derive from common hematopoietic stem cells. They can be classified into two in accordance to organs to be later differentiated; myeloid cells to be differentiated in bone marrow and lymphoid lineage cells to be differentiated in the thymus.
5. Immune tolerance -- is the process by which the immune system does not attack an antigen.
6. Cell adhesion molecules -- are proteins located on the cell surface involved with the binding with other cells or with the extracellular matrix (ECM)
7. Transcription factor -- is a protein that binds to specific DNA sequences, thereby controlling the flow (or transcription) of genetic information from DNA to mRNA..
8. Gut flora -- More than 100 species and 10 trillion bacteria are in the intestines. Using a portion of nutrition from the human or animal host, they form an ecosystem, harmonizing with other bacteria. This ecosystem is called gut flora consists of microorganisms that live in the digestive tracts of animals and is the largest reservoir of human flora, microbiota and microflora. The word microbiome is used.
Link : View original abstract at PNAS
