Researchers split the “AtoM” in search of a treatment for rheumatoid arthritis
Researchers at Osaka University discover a new type of bone-dissolving osteoclast that contributes to rheumatoid arthritis
Arthritis is a common chronic disease in which joints become inflamed, leading to stiffness and pain that can often be debilitating. Rheumatoid arthritis (RA) is an autoimmune form of the disease, arising when immune cells attack the tissue that lines the joints. There is a need for new treatment options, as current therapies only alleviate symptoms or, at best, slow the disease. Now, in a study published in Nature Immunology , researchers at Osaka University have discovered a previously unknown type of RA-causing cell within arthritic joints that could someday be a target for new treatments.
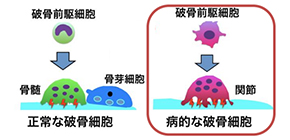
There are two major cellular culprits that contribute to RA. The first are immune cells, which release inflammatory chemicals around the tissue of affected joints. The second are osteoclasts, specialized cells that secrete acids and enzymes to break down bone. Osteoclasts normally help to remodel healthy bone, but in RA their bone-dissolving ability goes into overdrive and damages joints.
“The disease-modifying anti-rheumatic drugs available today predominantly act against the inflammatory response of immune cells,” says Masaru Ishii, professor at Osaka University Graduate School of Medicine and corresponding author of the study. “Therapies targeting osteoclasts are limited, largely because we don’t know enough about the osteoclasts involved in RA. We were interested in understanding whether these cells are somehow different from the osteoclasts involved in normal physiological processes.”
Osteoclasts are elusive, residing along the surface of bone under layers of cartilage and tissue. This makes them difficult to isolate in the lab, even with tractable models like mice. To collect the cells, the research group had to develop a surgical technique that allowed them to extract osteoclasts from the femurs of arthritic mice. With osteoclasts securely in hand, they were then able to gather new insights into RA.
“We tracked precisely how arthritis-inducing osteoclasts develop from their undifferentiated precursor cells,” explains Tetsuo Hasegawa, lead author of the study. “While normal osteoclasts are derived from stem cells in the bone marrow, we found that osteoclasts involved in RA come from blood-borne precursors. The circulating precursors enter the joint and differentiate into a unique sub-type of osteoclasts, which are larger and have distinct markers that aren’t seen in other osteoclasts.”
The newly discovered cells, dubbed “AtoMs” ( A r t hritis-associated o steoclastogenic M acrophages), have properties that could be exploitable in the search for new treatments. In one example highlighted by the study, the researchers found that AtoMs have high levels of a protein (called FoxM1) known to make cells invade nearby tissue. They speculated that by getting rid of this hallmark protein—splitting the AtoM, if you will—they could perhaps quell its arthritic tendencies. This is indeed what they found: when FoxM1 was chemically or genetically disrupted in AtoMs, arthritic mice showed reduced bone destructions in their joints.
“Our findings suggest that osteoclasts involved in RA have distinct properties that make them amenable to therapeutic targeting,” says co-author Masaru Ishii. “While there is still a lot to learn about this class of cells, we believe the discovery could open the door to new avenues of treatment.”
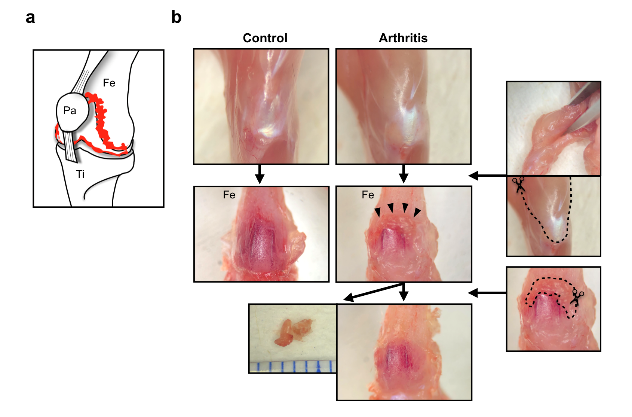
Fig. 1. Protocol for isolating joint tissue in arthritic mice
a, Schematic showing that hypertrophied joint tissue exists behind the knee ligament. b, Protocol for removing muscles and isolating joint tissue
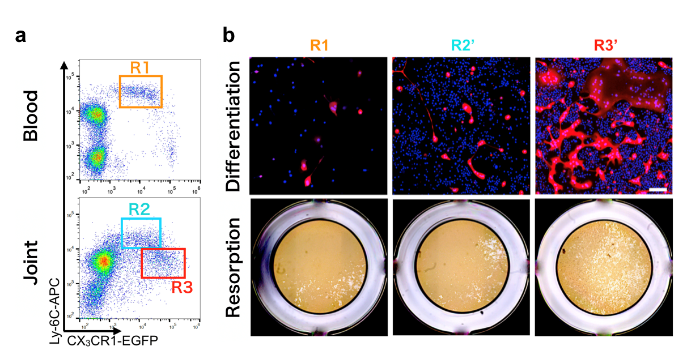
Fig. 2. Identification of arthritis-associated osteoclast precursor cells
a, Arthritis-associated osteoclast precursor cells (R3) exist only in the joint tissue and b, have the capacity to form osteoclasts and resorb bone

Fig. 3. Inhibition of Foxm1 suppresses osteoclast formation of arthritis-associated osteoclast precursor cells
The article, “Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1” was published in Nature Immunology at DOI: https://doi.org/10.1038/s41590-019-0526-7 .
Related links
