How fat could help in tailoring cancer treatment plans
Researchers from Osaka University identify a link between fat accumulation in liver tumors and patient response to immunotherapy
In recent years, the immunotherapy field has revolutionized oncology treatment methods by developing therapeutics that help a cancer patient’s own immune system fight their disease. However, these treatments have had varied efficacies in different cancer types. Although immunotherapy has become the standard therapeutic method for fighting liver cancer, also called hepatocellular carcinoma (HCC), its response rate has only been approximately 30% in patients with advanced disease. In a recent article published in Hepatology, a team led by researchers at Osaka University developed an analytical method that could help predict if an HCC patient would successfully respond to immunotherapy.
The tumor immune microenvironment (TIME) is the area inside the tumor in which various immune cells and other cell types interact and send/receive messages and signals. Within the TIME, the presence of tumor-infiltrating lymphocytes, one type of white blood cells, is critical because they can recognize tumor cells and help fight them. Additionally, expression levels of immune checkpoint proteins are also important, because these are targets of many immunotherapy drugs. Therefore, the Osaka University group aimed to examine and characterize the TIMEs of various HCC patients to identify immunotherapy-susceptible cases.
“We examined 113 patients that developed HCC without hepatitis virus infection,” says Hiroki Murai, lead author of the study. “We used a multi-omics approach, meaning we examined the DNA sequences of specific genes frequently mutated in HCC, but also performed a more general assessment of RNA sequences to investigate expression levels of all genes in these tumors.”
Using this workflow, the team categorized the 113 HCC cases based on their TIME composition estimated by gene expression profile. The TIME is commonly labeled as immune-excluded, immune-active, or immune-exhausted. Interestingly, they found a unique TIME in 23% of the HCC cases that had a significant amount of intra-tumoral fat accumulation, known as steatotic HCC.
“Steatotic HCC tumors tended to have an immune-enriched, but immune-exhausted TIME,” explains senior author Tetsuo Takehara. “There was high infiltration of immune cells, including M2 macrophages and cancer-associated fibroblasts, but the nearby T cells were exhausted. They lost much of their fighting ability.”
The group also determined that the increased fat levels in these tumor cells induced expression of PD-L1, an immune checkpoint molecule that leads to an immunosuppressive TIME.
“PD-L1 is a common target of immunotherapy drugs because interfering with this protein can help restore the functions of the immune cells,” says Takahiro Kodama, co-lead author of the study. “Our findings suggest that HCC tumors with steatosis would therefore be susceptible to these types of drugs.”
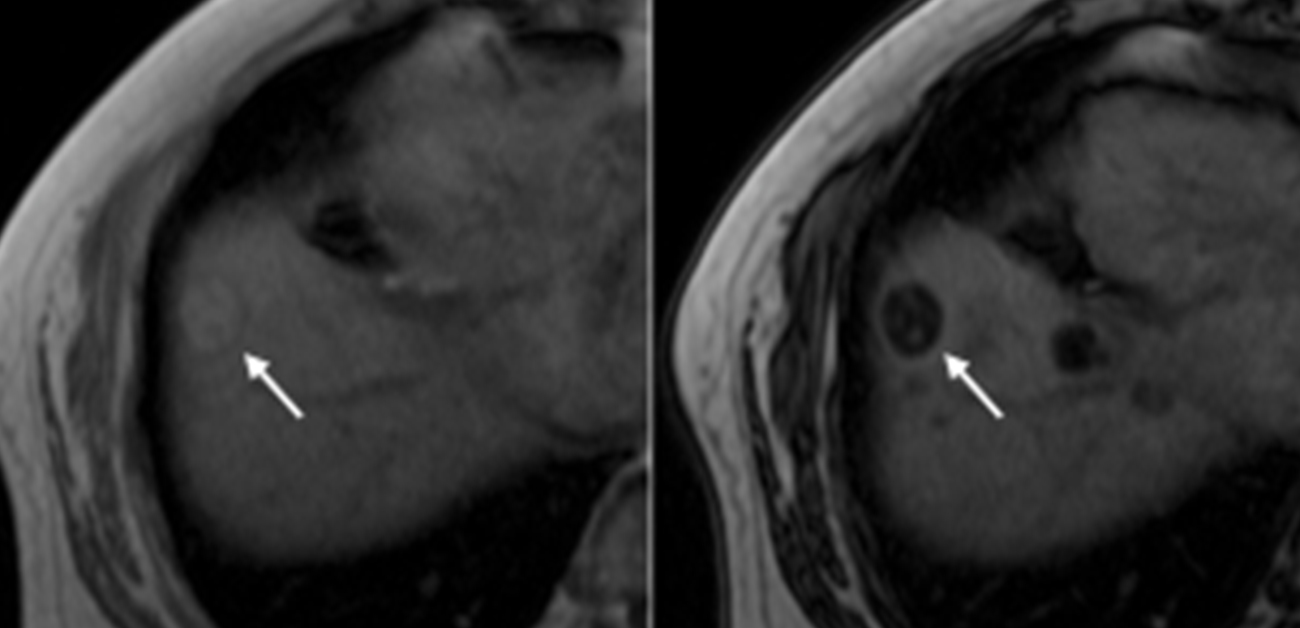
Searching for a non-invasive identification system, the authors found that MRI scans could be used to identify steatotic HCC patients who may be susceptible to a combined immunotherapy approach. The data presented in this work are therefore extremely important and demonstrate that intratumor steatosis could be used as a novel biomarker for evaluating immunotherapy efficacy in HCC cases.
Figure 1. Quantification of intratumoral fat accumulation using MRI images predicts the therapeutic efficacy of combined immunotherapy for hepatocellular carcinoma (HCC).
(credit: Takahiro Kodama)
Figure 2. (A) Hematoxylin and eosin (HE) staining image of steatotic hepatocellular carcinoma (HCC), (B) strong immune cell infiltration in steatotic HCC, (C) strong immune exhaustion of infiltrated cells in steatotic HCC.
(CC BY. 2022 Hiroki Murai et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology)
Figure 3. (A-B) MRI images of steatotic hepatocellular carcinoma (HCC) (arrows) (A1; in-phase T1-weighted gradient-echo image showing a hyperintense mass; A2; Opposed-phase T1-weighted gradient-echo image showing decreased signal intensity of the tumor; A3; Hepatic artery phase of Gd-EOB-DTPA contrast-enhanced image showing arterial enhancement of the tumor, A4; 20-minute hepatobiliary phase of Gd-EOB-DTPA contrast-enhanced image reveals a drop in the signal intensity of the tumor); (B) hematoxylin and eosin (HE) staining images (B). (C) Histologically quantified intratumoral fat droplet content strongly correlates with fat content calculated from MRI images; (D) Progression-free survival (PFS) is significantly prolonged with atezolizumab/bevacizumab combination therapy in steatotic HCC.
(CC BY. 2022 Hiroki Murai et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology)
The article, “Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma,” was published in Hepatology at DOI: https://doi.org/10.1002/hep.32573.
