A library of mice to look up the best liver cancer treatment
Researchers from Osaka University find that certain liver cancer subtypes identifiable by a serum biomarker are especially susceptible to treatment
If you want to get smarter, the library is a good place to start. And for cancer researchers, smarter treatment selection for patients may now start with a library of cancer genes.
In a study published this month in Clinical Cancer Research, a journal of the American Association for Cancer Research, researchers from Osaka University have developed a technique to study a library of genes in lab mice—rather than one specific gene at a time—to identify which cancer genes drive specific liver cancers. There’s hope that this can in turn be used to improve prognostics and guide treatment selection for this deadly cancer.
Hepatocellular carcinoma (HCC), a type of cancer that starts in the liver, is difficult to detect early and to treat, making it one of the top causes of cancer death worldwide. Treatments for HCC do exist, but some work better than others for different people, and it’s hard to know which one to choose. But now, researchers from Japan have developed a method using lab mice that can indicate which liver cancers are driven by specific cancer genes—and what’s more, they’ve found a biomarker that could point out people who will benefit the most from a common HCC treatment.
To do this, the researchers first took ten cancer genes that are known to be involved in the pathways leading to HCC and used them to build a DNA “library” by injecting them into lab mice. When the mice developed liver tumors, sequencing the tumor genomes showed which tumors were associated with which genes in the library. Then, mice with liver cancer were treated with the chemotherapy drug lenvatinib to indicate which cancer genes in the library produce tumors that the treatment works particularly well against.
“Our results suggest that FGF19-driven HCC, which generally carries a poor prognosis, may be susceptible to lenvatinib,” says Takahiro Kodama, lead author of the study. “But beyond that specific finding, the technique we used could be used to assess drug susceptibility for other genetic drivers as well.”
Knowing which gene drives a cancer is powerful information, but to make it useful in the real world of cancer treatment, there needs to be an easy way to identify when patients have FGF19-driven HCC. Working in liver cells in the lab, the research team identified six proteins whose expression was decreased when the FGF19 gene was turned off. From these six, the team determined that a protein known as ST6GAL1 was the most closely correlated with FGF19 in HCC. Further testing of biosamples from actual liver cancer patients confirmed that serum levels of ST6GAL1 could be used to identify patients with FGF19-driven HCC with a high level of sensitivity and specificity.
“While some HCC mouse models already exist, our system can be used to study any gene set, and eliminates the need for expensive and time-consuming genetic studies done one-by-one in individually prepared mice,” says Tetsuo Takehara, senior author of the study. “This new model may be a valuable tool for preclinical drug assessment and increasing the efficacy of drug therapy.
The use of this model to show that FGF19-driven liver cancer is susceptible to lenvatinib treatment, and further to point to a specific protein that could be used as a biomarker, shows the potential of this novel technique.
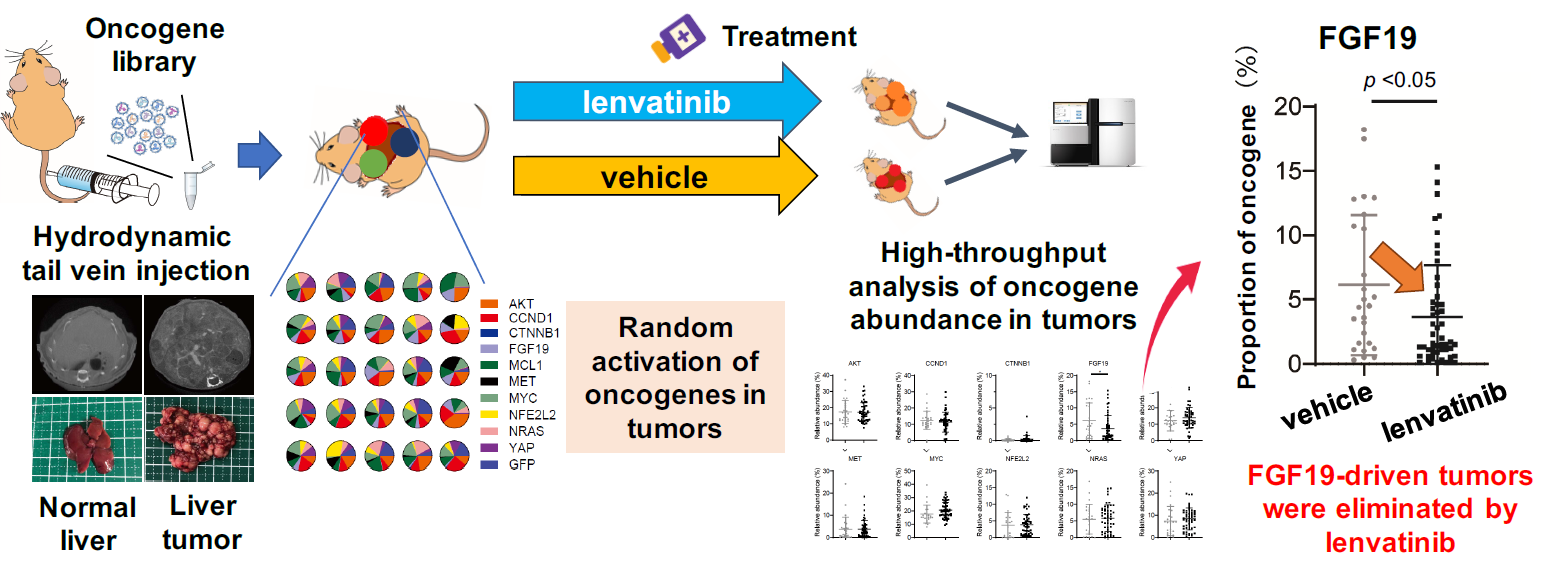
Figure 1. The novel mouse model that reproduces the inter-tumor oncogene heterogeneity of liver tumors and discovery of high susceptibility of FGF19-driven tumors to lenvatinib treatment
By simultaneously introducing multiple oncogenes into hepatocytes, we created a new mouse model that randomly activates these oncogenes to develop liver cancer with high intertumor heterogeneity. Applying lenvatinib treatment to this model significantly reduced the proportion of tumors expressing the FGF19 gene compared to the untreated group, revealing that liver cancer with high FGF19 expression is highly sensitive to lenvatinib.
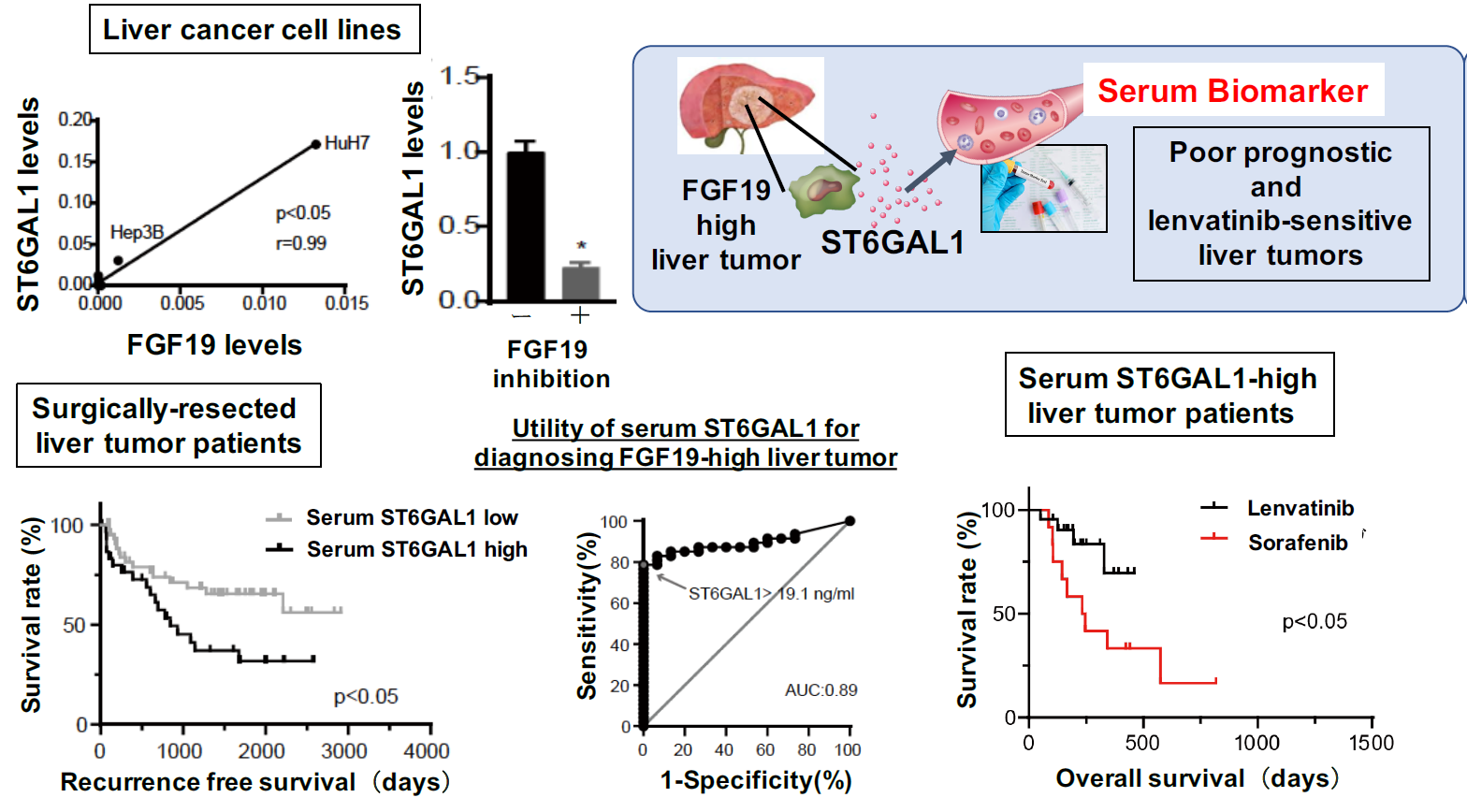
Figure 2. Serum ST6GAL1 is a novel biomarker for predicting poor prognostic and lenvatinib-sensitive liver cancer
Proteome analysis using a liver cancer cell line identified a secretory protein, ST6GAL1, whose expression is regulated by FGF19 (Upper left). Serum ST6GAL1 levels can be used to identify liver cancer cases with high expression of FGF19 and poor prognosis (Lower left). When patients were stratified based on serum ST6GAL1 concentration, we found that in the high-ST6GAL1 group, overall survival among lenvatinib-treated patients was significantly longer than for the sorafenib-treated group (Lower right). Taken together, serum ST6GAL1 is a novel biomarker for identifying certain poor-prognosis liver cancers for which lenvatinib may be more effective.
The article, “ST6GAL1 is a novel serum biomarker for lenvatinib-susceptible FGF19-driven hepatocellular carcinoma,” was published in Clinical Cancer Research at DOI: https://doi.org/10.1158/1078-0432.CCR-20-3382
