A simple blood test to identify patients at risk of nonalcoholic fatty liver disease
Researchers at Osaka University identify a new noninvasive biomarker that can be used in the diagnosis of nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and can progress to liver cirrhosis, liver failure or cancer. Currently, nonalcoholic steatohepatitis (NASH) diagnosis requires an invasive liver biopsy which can lead to procedural complications. Now, researchers at Osaka University working with international collaborators have identified a noninvasive biomarker that can identify patients at risk of NAFLD complications using a simple blood test.
Owing to the increasing prevalence of obesity worldwide, as many as one in four humans has NAFLD. Unrelated to alcohol intake by definition, the early stage – NAFL (nonalcoholic fatty liver) – is asymptomatic. Unfortunately, progression to NASH incurs inflammatory damage and eventually liver fibrosis occurs; this may further lead to adverse outcomes. Liver deterioration can be deferred by lifestyle modifications comprising diet and exercise; therefore, early diagnosis is key.
Diagnostic confirmation requires a needle biopsy; however, the disadvantages include expense and variability in sampling and interpretation. The research team investigated whether they could devise a diagnostic screen using transcriptomics, the emerging science of analyzing the ‘transcriptome,’ the entire array of an organism’s messenger RNA molecules derived from expression of the genome.
“We obtained liver tissue from over 300 Japanese and European patients with biopsy-proven NAFLD and performed global RNA sequencing,” co-first author Kazuhiro Kozumi explains. “Remarkably, from the protein patterns we could not only distinguish NASH from NAFL, but also determine the molecular hallmarks of NASH pathology. Specifically, we pinpointed that levels of thrombospondin-2 (TSP-2), a glycoprotein encoded by the THBS2 gene, were increased in both NASH and advanced fibrosis.”
The researchers established that THBS2 expression in liver cells paralleled the clinical indicators conventionally used to categorize the pathological changes including serum enzyme levels, NAFLD Activity Score and NAFLD Fibrosis Score. “Serum levels of TSP-2 in NAFLD patients were significantly higher in NASH than in NAFL,” co-first author Takahiro Kodama claims, “and, interestingly, the increase tallied with the degree of fibrosis.”
Corresponding author Tetsuo Takehara explains the clinical relevance of their research findings. “Both hepatic THBS2 gene expression in the liver and serum protein levels of TSP-2 can diagnose cases of NASH and/or advanced fibrosis. A simple and convenient blood test can provide a clinically useful early warning system for complications of NAFLD and inform lifestyle modifications or other interventions that may alter the course of the disease and improve the prognosis.”
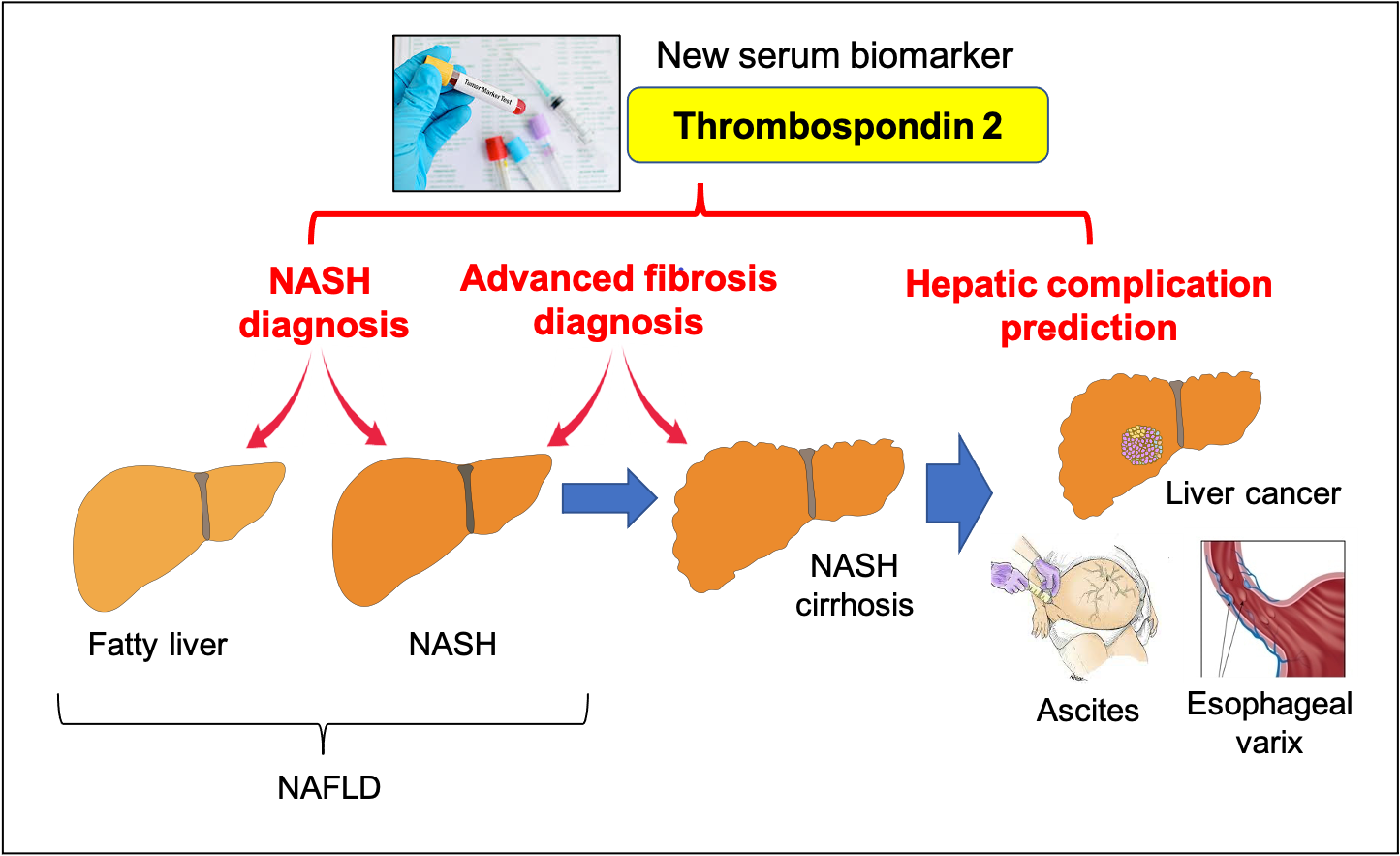
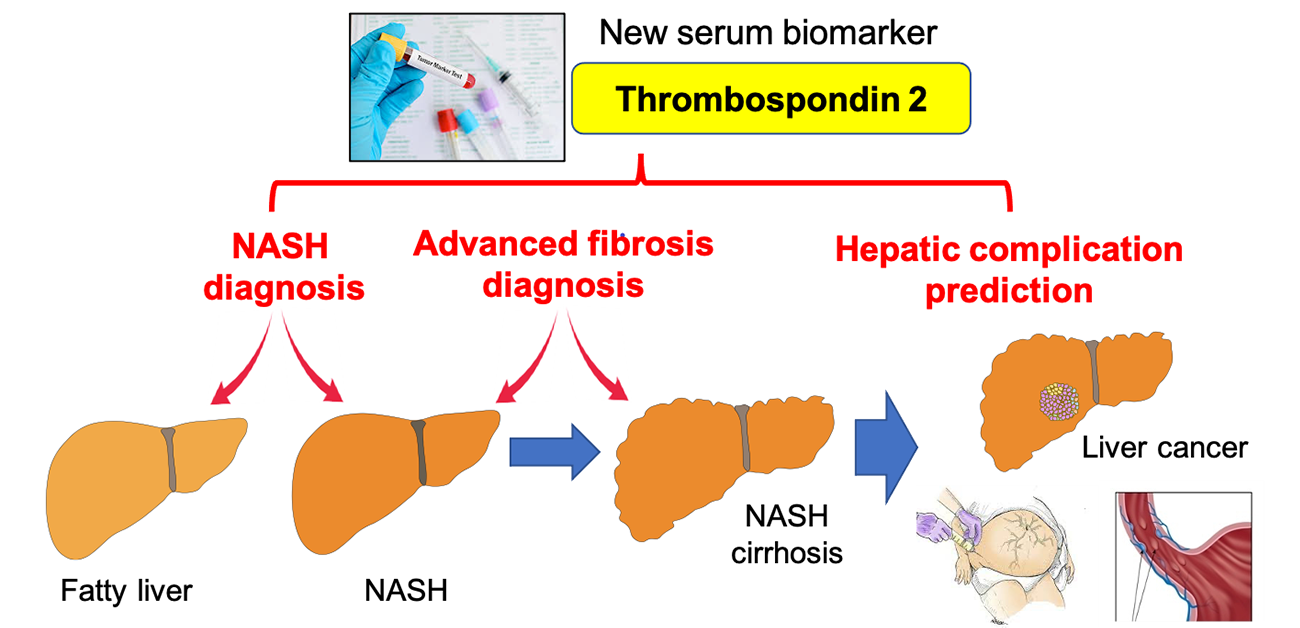
Figure 1. Thrombospondin 2 is a new diagnostic and prognostic serum biomarker in NAFLD
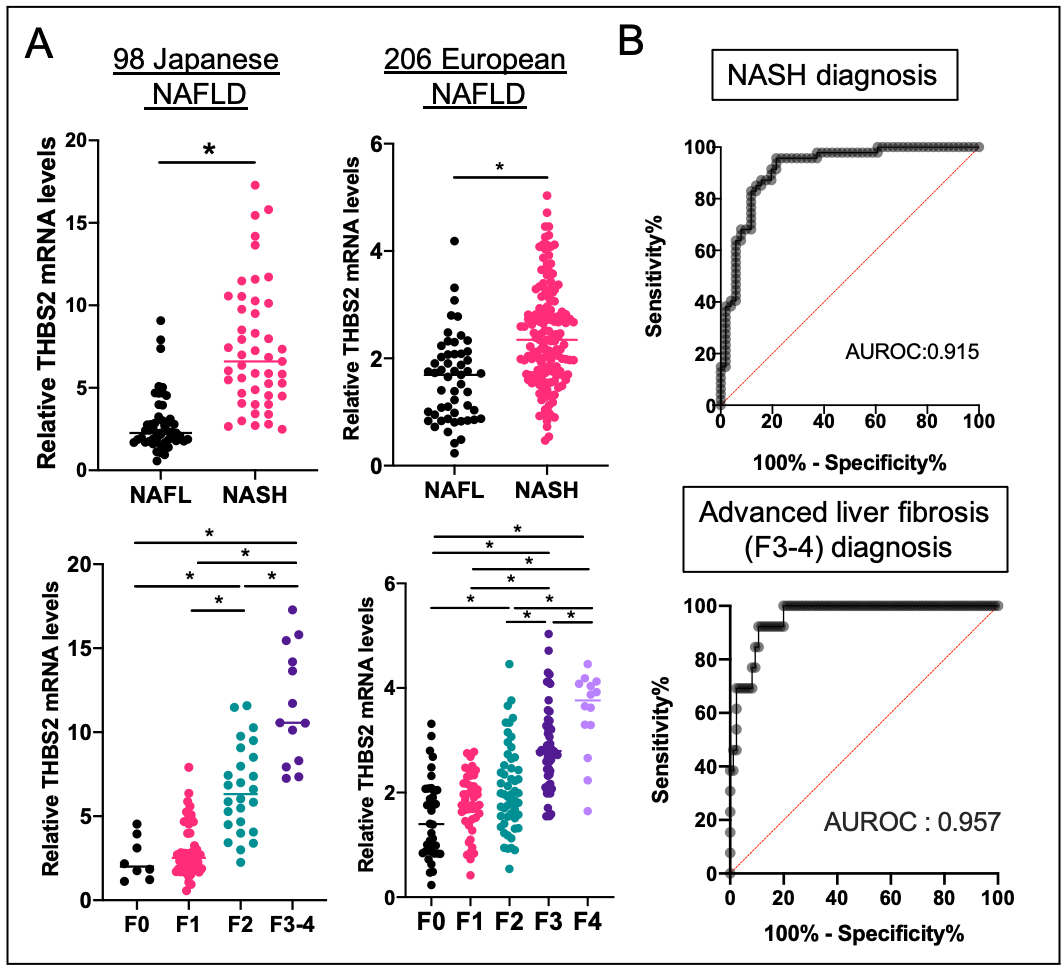
Figure 2. Hepatic THBS2 gene expression and clinical stage in patients with NAFLD
A) Hepatic THBS2 mRNA levels are elevated in the NASH group and increases in parallel with liver fibrosis stage, * p<0.05
B) Hepatic THBS2 mRNA levels can diagnose cases of NASH and advanced fibrosis
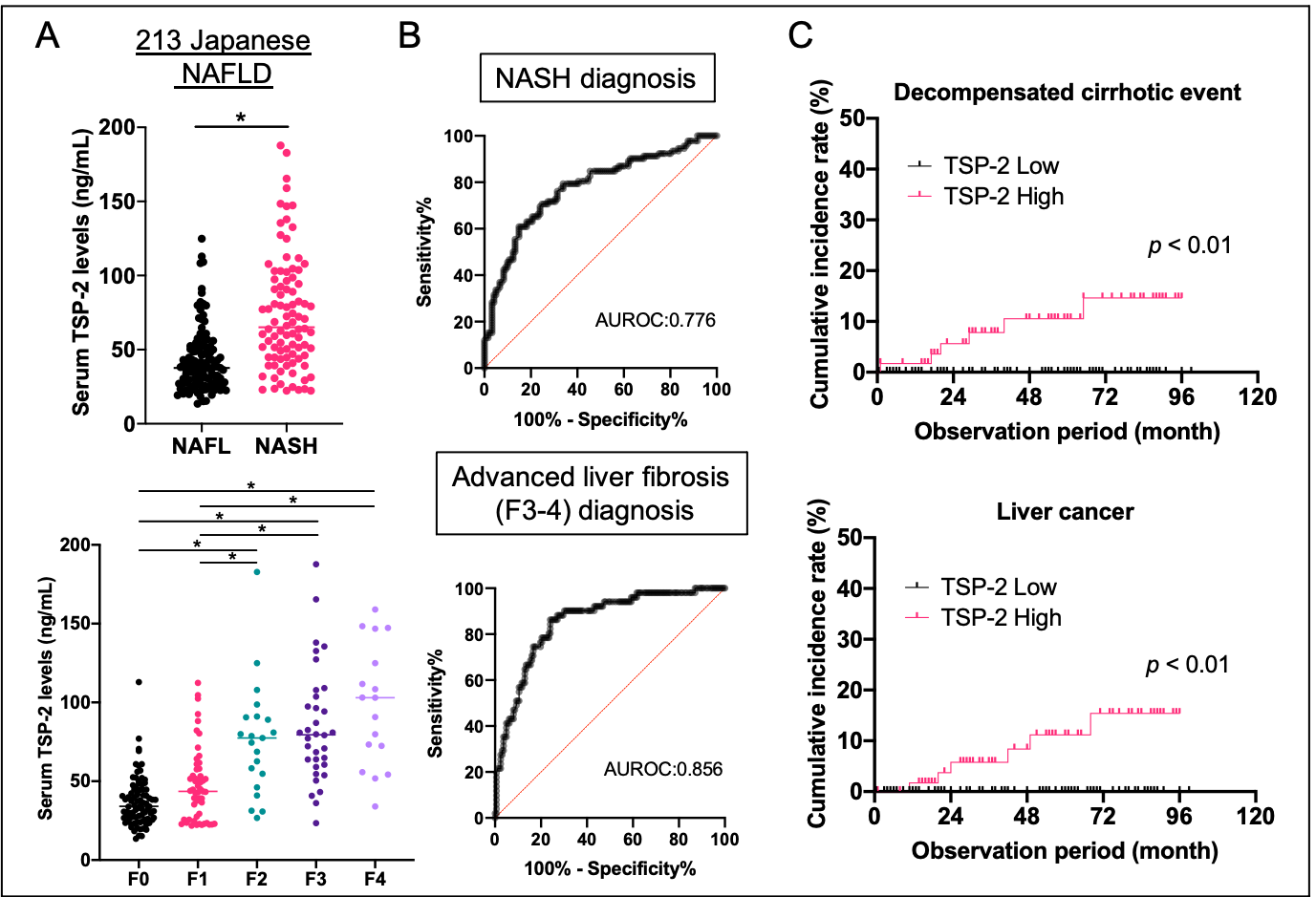
Figure 3. Serum TSP-2 level and clinical stage in patients with NAFLD
A) Serum TSP-2 levels are elevated in the NASH group and increase in parallel with liver fibrosis stage, * p<0.05
B) Serum TSP-2 levels can diagnose cases of NASH and advanced fibrosis
C) High serum TSP-2 group has significantly higher incidence of complications associated with liver cirrhosis and liver cancer.
The article, “Transcriptomics identify thrombospondin-2 as a biomarker for nonalcoholic steatohepatitis and advanced liver fibrosis” was published in Hepatology at DOI: https://doi.org/10.1002/hep.31995.
