Structure of MotA molecule of the flagellar motor with torque comparable to Formula One engine elucidated
Will contribute to the design of artificial biological nanomachines, a dream technology
A group led by Professor HONMA Michio and Researcher TAKEKAWA Norihiro at the Graducate School of Science, Nagoya University; and Professor NAMBA Keiichi, Assistant Professor KATO Takayuki, and Specially Appointed Assistant Professor TERAHARA Naoya at the Graduate School of Frontier Biosciences, Osaka University, clarified the 3D structure of a MotA molecule, one of the bacterial flagellar motor proteins, through image analysis using electronic microscopy. The characteristic molecular structure of a MotA protein, which plays a key role in movement of the motor, was also clarified. (TAKEKAWA Norihiro currently serves as a researcher at Osaka University.)
A bacterial flagellar motor, which is built from various kinds of parts in its cell, has been drawing attention in various fields such as medicine and mechanic engineering as a biological nanomachine. The flagellar motor is 50 nm or less in size and operates at a speed of 200 ~ 1,000 rps. These nanomachines cannot be artificially created by current scientific capability because the structure of the components of these motors is unknown.
The drive part of a flagellar motor consists of two parts: a rotor and a stator. The energy source moving the flagellar motor is an ion influx from the outside of the cell into the inside of the cell. Ions flow through the channel in the stator and convey their power to the rotor, through which the power is converted into the motor’s turning force.
This group determined stable MotA proteins by comparing properties of MotA proteins created from various bacteria using genetic recombination technology. As a result, MotA derived from hyperthermophilic bacterium Aquifex aeolicus was found to be stably and abundantly generated because of its properties, so this group purified MotA. A size exclusion chromatography and chemical crosslinking experiments revealed that MotA alone formed a stable tetramer.
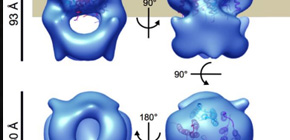
Furthermore, this group clarified the 3D structures of a MotA complex by analyzing thousands of electronic microscope images of the MotA complex. It was found that the structure of the MotA complex was composed of two parts: a slightly elongated globular part and a pair of arch-like domains with spiky projections. This group clarified the 3D structure of these parts, a kind of heart of this biological nanomachine.
It is assumed that the characteristic structure of the MotA discovered in this study is important in generating motor torque with high energy conversion efficiency. Clarification of a mechanism for clean energy conversion of living organisms will be greatly helpful in designing artificial nanomachines.
Abstract
Rotation of bacterial flagellar motor is driven by the interaction between the stator and rotor, and the driving energy is supplied by ion influx through the stator channel. The stator is composed of the MotA and MotB proteins, which form a hetero-hexameric complex with a stoichiometry of four MotA and two MotB molecules. MotA and MotB are four- and single-transmembrane proteins, respectively. To generate torque, the MotA/MotB stator unit changes its conformation in response to the ion influx, and interacts with the rotor protein FliG. Here, we overproduced and purified MotA of the hyperthermophilic bacterium Aquifex aeolicus. A chemical crosslinking experiment revealed that MotA formed a multimeric complex, most likely a tetramer. The three-dimensional structure of the purified MotA, reconstructed by electron microscopy single particle imaging, consisted of a slightly elongated globular domain and a pair of arch-like domains with spiky projections, likely to correspond to the transmembrane and cytoplasmic domains, respectively. We show that MotA molecules can form a stable tetrameric complex without MotB, and for the first time, demonstrate the cytoplasmic structure of the stator.
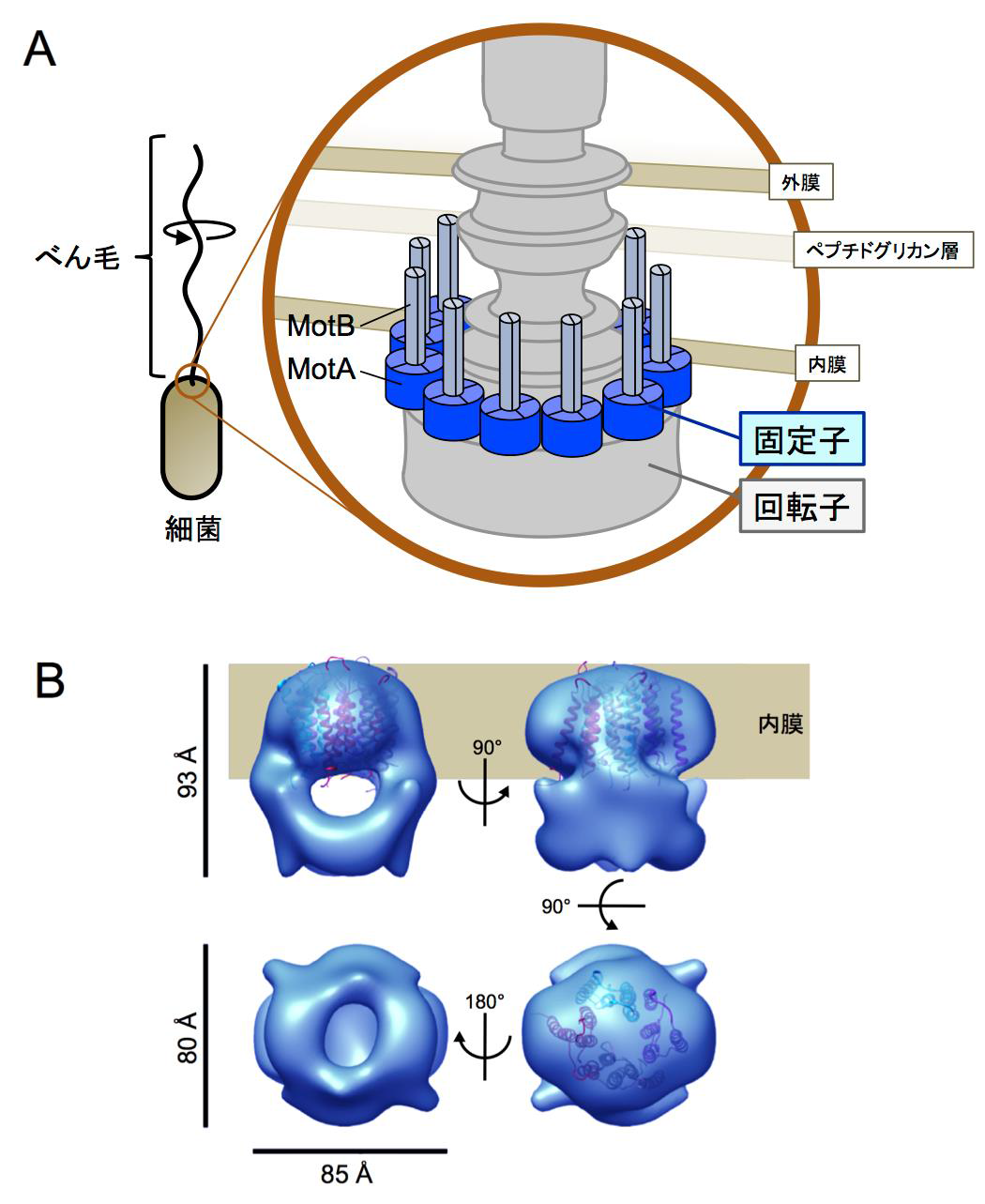
Figure 1
To learn more about this research, please view the full research report entitled “ The tetrameric MotA complex as the core of the flagellar motor stator from hyperthermophilic bacterium ” at this page of the Scientific Reports website.
Related link
