Lipid absorption mechanism by protein TTC39B clarified
A step forward to the development of innovative drugs for arterial sclerosis and non-alcoholic steatohepatitis (NASH)
Viral hepatitis, a major cause of hepatic cirrhosis and cancer, can now be cured by over-the-counter drugs. But on the other hand, non-alcoholic steatohepatitis (NASH) featuring ectopic lipid accumulation in the liver associated with metabolic syndrome due to obesity and visceral fat accumulation has gained prominent attention as the cause of hepatic cirrhosis and cancer. Thus the development of drugs effective for NASH is highly sought after.
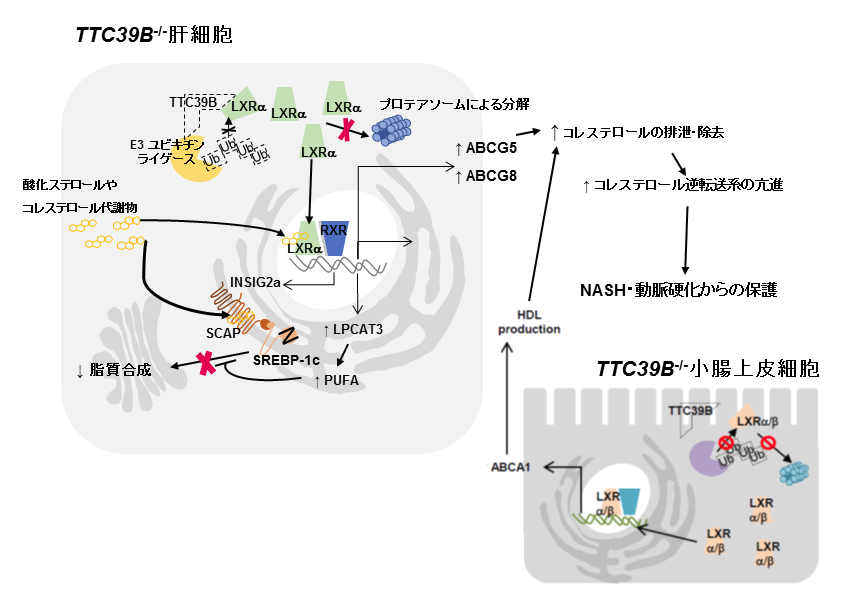
A group of researchers led by Assistant Professor KOSEKI Masahiro at the Department of Cardiovascular Medicine, Graduate School of Medicine, Osaka University, and Professor Alan R. Tall at Columbia University, by using mice models of NASH and arterial sclerosis, clarified that when the function of protein TTC39B was inhibited, the absorption and accumulation of cholesterol in the body was suppressed, a world first.
This group produced knockout mice in order to examine a molecular mechanism of TTC39B inhibition. In mice with NASH induced by diet and lacking TTC39, cholesterol accumulation and inflammatory cell infiltration were suppressed. And in arterial sclerosis models lacking low-density lipoprotein receptor (Ldlr) and TTC39, progression of arterial sclerosis and decreased fatty liver were observed. It was found that TTC39B inhibition decreased both cholesterol absorption on the intestinal epithelia cells and cholesterol accumulation in the liver and blood vessels.
At the molecular level, it was found that TTC39B inhibition brought about the suppressed ubiquitination of liver X receptor (LXR)α and reduced proteasomal degradation, which increased the amount of protein LXRα, activating target genes.
In chemical compounds synthesized for activating LXRα, SREBP-1c-related lipogenesis has been a problem. However, using by mice models of NASH and arterial sclerosis, this group clarified that phosphatidylcholine inhibited SREBP-1c processing in LXRα activation due to TTC39B inhibition, so that the lipogenesis was not promoted.
It is expected that this will lead to the development of innovative drugs for both NASH and arterial sclerosis by inhibition of TTC39B.
Abstract
Cellular mechanisms that mediate steatohepatitis, an increasingly prevalent condition in the Western world for which no therapies are available 1 , are poorly understood. Despite the fact that its synthetic agonists induce fatty liver, the liver X receptor (LXR) transcription factor remains a target of interest because of its anti-atherogenic, cholesterol removal, and anti-inflammatory activities. Here we show that tetratricopeptide repeat domain protein 39B (Ttc39b, C9orf52) (T39), a high-density lipoprotein gene discovered in human genome-wide association studies 2 , promotes the ubiquitination and degradation of LXR. Chow-fed mice lacking T39 (T39 −/− ) display increased high-density lipoprotein cholesterol levels associated with increased enterocyte ATP-binding cassette transporter A1 (Abca1) expression and increased LXR protein without change in LXR messenger RNA. When challenged with a high fat/high cholesterol/bile salt diet, T39 −/− mice or mice with hepatocyte-specific T39 deficiency show increased hepatic LXR protein and target gene expression, and unexpectedly protection from steatohepatitis and death. Mice fed a Western-type diet and lacking low-density lipoprotein receptor (Ldlr −/− T39 −/− ) show decreased fatty liver, increased high-density lipoprotein, decreased low-density lipoprotein, and reduced atherosclerosis. In addition to increasing hepatic Abcg5/8 expression and limiting dietary cholesterol absorption, T39 deficiency inhibits hepatic sterol regulatory element-binding protein 1 (SREBP-1, ADD1) processing. This is explained by an increase in microsomal phospholipids containing polyunsaturated fatty acids, linked to an LXRα-dependent increase in expression of enzymes mediating phosphatidylcholine biosynthesis and incorporation of polyunsaturated fatty acids into phospholipids. The preservation of endogenous LXR protein activates a beneficial profile of gene expression that promotes cholesterol removal and inhibits lipogenesis. T39 inhibition could be an effective strategy for reducing both steatohepatitis and atherosclerosis.
To learn more about this research, please view the full research report entitled “ TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis ” at this page of the Nature website.
Related link
