Host factors that trigger differentiation of malaria Plasmodium clarified
Existing drugs could prevent malaria
A group of researchers led by YAMAMOTO Masahiro from the Research Institute for Microbial Diseases at Osaka University clarified that the host factor C-X-C chemokine receptor type 4 (CXCR4) was necessary for differentiation of liver-stage malaria Plasmodium into exoerythrocytic forms (EEFs).
Malaria is caused by infection with protozoan parasites that belong to the genus Plasmodium . The malaria Plasmodium has a three-stage life cycle: 1. the vector stage in the mosquito, 2. the liver stage in which sporozoites from a mosquito’s salivary glands are released into the mammalian host’s liver, and 3. the blood stage in which clinical malaria disease is caused in the host.
During the liver stage, malaria parasites form a parasitophorous vacuole (PVs) in the finally-invaded hepatocytes, in which the rod-shaped sporozoites of Plasmodium transform into spherical EEFs via bulbous expansion, eventually differentiating into merozoites. However, the molecular mechanisms underlying this morphological transformation were unknown.
Focusing on the fact that morphological transformation of the rod-shaped sporozoites into spherical EEFs depends on protein kinase C (PKC), they carried out research to find the following:
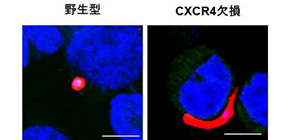
- 1. In hepatocytes, both the Plasmodium berghei rodent malaria parasite and the Plasmodium falciparum human malaria parasite, CXCR4-dependently transform into spherical exoerythrocytic forms (EEFs), generating merozoites.
- 2. The expression of CXCR4 elevates intracellular Ca 2+ levels in hepatocytes, thereby triggering sporozoite transformation into EEFs.
- 3. In mice to which CXCR4 inhibitors were administered in advance, the onset of malaria is suppressed and the survival rate improves.
These results showed that Ca determined differentiation of sporozoites into merozoites. The researchers suggested that rod-shaped sporozoites that invaded hepatocytes induced CXCR4, elevating intracellular Ca 2+ levels in hepatocytes, thereby triggering sporozoite transformation into EEFs, and subsequently becoming spherical merozoites. Thus, they clarified that CXCR4 was the host factor that played a decisive role in sporozoite differentiation into EEFs during the liver stage of Plasmodium development.
Currently marketed CXCR4 inhibitors will become a promising prophylaxis for malaria, which is rampant in developing countries in Africa, Southeast Asia, and South America.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
The article, "CXCR4 regulates Plasmodium development in mouse and human hepatocytes" was published in the Nature at DOI: https://doi.org/ 10.1084/jem.20182227.
Related links
