Innate immune cells abnormally activated and induced development of Th17 cells
Step in the understanding and therapeutic treatment of inflammatory bowel diseases
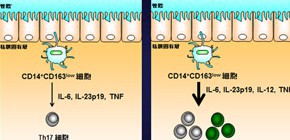
Under the combined leadership of Professor Kiyoshi TAKEDA (Laboratory of Immune Regulation, Graduate School of Medicine, Osaka University), Assistant Professor Jun-ichi NISHIMURA and Professor Masaki MORI (both of the Division of Gastroenterological Surgery, Department of Surgery, Osaka University), researchers have clarified a possible role of human intestinal innate immune cells in inducing the development of Th17 cells, cells implicated in the pathogenesis of autoimmune and inflammatory diseases, including Crohn's disease (CD). That is to say, this group clarified that innate immune cells (specifically, intestinal lamina propria cells [LPCs]) from patients with CD did abnormally activate and induce Th17 cell development.
In Japan, some 30,000 people are affected with CD and it is designated as one of the diseases treated under The Ministry of Health Research Project for the Treatment of Special Chronic Diseases. Further research will lead to possible therapeutic treatment for CD and other inflammatory bowel diseases.

Abstract
Background & Aims
Abnormal activity of innate immune cells and T-helper (Th) 17 cells has been implicated in the pathogenesis of autoimmune and inflammatory diseases, including Crohn’s disease (CD). Intestinal innate immune (myeloid) cells have been found to induce development of Th17 cells in mice, but it is not clear if this occurs in humans or in patients with CD. We investigated whether human intestinal lamina propria cells (LPCs) induce development of Th17 cells and whether these have a role in the pathogenesis of CD.
Methods
Normal intestinal mucosa samples were collected from patients with colorectal cancer and noninflamed and inflamed regions of mucosa were collected from patients with CD. LPCs were isolated by enzymatic digestion and analyzed for expression of HLA-DR, lineage markers CD14 and CD163 using flow cytometry.
Results
Among HLA-DRhigh Lin− cells, we identified a subset of CD14+ CD163low cells in intestinal LPCs; this subset expressed Toll-like receptor (TLR) 2, TLR4, and TLR5 mRNAs and produced interleukin (IL)-6, IL-1β, and tumor necrosis factor in response to lipopolysaccharide. In vitro co-culture with naïve T cells revealed that CD14+ CD163low cells induced development of Th17 cells. CD14+ CD163low cells from inflamed regions of mucosa of patients with CD expressed high levels of IL-6, IL-23p19, and tumor necrosis factor mRNAs, and strongly induced Th17 cells. CD14+ CD163low cells from the noninflamed mucosa of patients with CD also had increased abilities to induce Th17 cells compared with those from normal intestinal mucosa.
Conclusions
CD14+ CD163low cells in intestinal LPCs from normal intestinal mucosa induce differentiation of naive T cells into Th17 cells; this activity is increased in mucosal samples from patients with CD. These findings show how intestinal myeloid cell types could contribute to pathogenesis of CD and possibly other Th17-associated diseases.
Figure 1

Figure 2
To learn more about this research, please read the full research report entitled "Increased Th17-Inducing Activity of CD14+ CD163 low Myeloid Cells in Intestinal Lamina Propria of Patients with Crohn’s Disease" at this page of the Gastroenterology website.
Related link :

