CO2-induced transitions between paramagnetism and ferrimagnetism could be used for sensing devices
A group of researchers of Tohoku University and Osaka University has developed a porous magnet that converts from ferrimagnetic to paramagnetic on CO2 adsorption and returns to the ferrimagnetic state on CO2 desorption, using CO2-responsive magnetic metal–organic framework (MOF) materials.
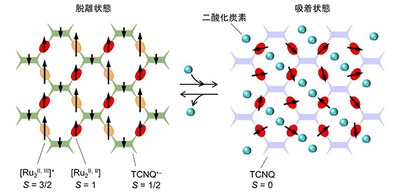
When CO2 is adsorbed between layers of a MOF, the framework undergoes a magnetic and structural phase transition that involves an intralattice electron transfer, converting a ferrimagnetic MOF to a paramagnetic form.
Ferrimagnetism is a kind of magnetism where magnetic moments have opposing moments, but the antiparallel moments do not cancel each other out, and a spontaneous magnetization occurs. Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field.
In recent years, magnet with improved functionality not realized by conventional magnet, and multifunctional magnetic materials, in which magnetism is co-existing and/or coupled with a second property of interest, are in demand. Utilizing molecular flexibility enables multifunctional magnets such as multiferroics.
This group focused on molecular porous materials MOFs, which consist of metal ions/clusters and organic ligands. Since framework flexibility is one of the most important characteristics of MOFs, they are used for a variety of applications. MOF’s modular designability due to the vast diversity of metal ions and organic ligands enables the strategic development of multifunctional magnet.
In porous magnet, a MOF can adsorb small molecules such as organic solvent and water in the pores they form and can desorb these molecules while maintaining the framework. Using the MOF, this group has developed the magnet which changes the magnetic phase transition temperature through solvent adsorption/desorption and realized the magnetic switching by oxygen adsorption/desorption.
The magnetic phase change from a ferrimagnet to an antiferromagnet achieved by the insertion of free oxygen molecules was an innovative achievement due to oxygen’s paramagnetic properties.
Since atmospheric gases (such as nitrogen, argon, and CO2) were diamagnetic and only interacted weakly with MOFs, it was thought difficult to develop matter that the magnet’s properties (magnetic phase) was transformed by the adsorption and desorption of molecules.
In this study, however, the layered molecular magnet was ferrimagnetism (its magnetism was ‘switched on’) before absorbing CO2 (phase transition temperature: Tc=110K). When CO2 was adsorbed to this molecular magnet at PCO2 < 5 kPa, a CO2-responsive magnetic MOF converted from ferrimagnetic to paramagnetic on CO2 adsorption, and returned to the ferrimagnetic state on CO2 desorption, which was reversible. This was caused by CO2-induced structural changes (the guest CO2 molecules accommodated in the framework) and a change in its electronic conductivity and permittivity.
MOFs are driven by external stimuli such as adsorption and desorption of molecules. This study demonstrated that magnetic ordering in MOFs could be controlled by adsorption and desorption of CO2, which will be applied to magnetic sensor of CO2. Porous magnets that undergo a magnetic phase transition in response to gaseous adsorbates will be desirable for the development of sensing devices.
The results of this study suggest that if MOFs are tuned, it is possible to control physical properties of porous materials by the presence or absence of guest molecules such as O2, N2, and CO2, within their pores. This group’s research results will expand the potential of property control by gas adsorption.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
The article " A metal–organic framework that exhibits CO2-induced transitions between paramagnetism and ferrimagnetism," appears in Nature Chemistry at DOI: https://doi.org/10.1038/s41557-020-00577-y.
