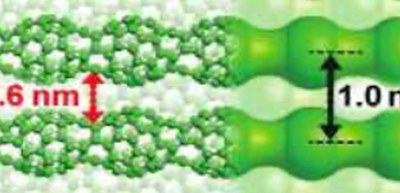
Immobilization and conversion of CO2 can be realized using the sub-nm space of 1D uneven-structured C60 polymer film
A group of researchers from Nagoya University and Osaka University discovered that H2O was produced at room temperature (RT) in the sub-nm space of 1D uneven-structured (peanut-shaped) C60 polymer (1D polymer) film. C60 is a carbon-based molecule that is made up of 60 carbon atoms with 20 hexagons and 12 pentagons.
Immobilization and reuse of carbon dioxide (CO2) are urgent tasks to suppress global warming and sustain the global environment. Nanoporous materials have been shown to exhibit storage, exchange, separation, and reaction fields when molecules and/or ions are incorporated. Immobilization and reuse of environmentally hazardous compounds, such as CO2, NOx, and SOx, using nanoporous materials, will play key roles in solving our environmental and energy issues. Furthermore, if these nanoporous materials exhibit high electrical conductivity or high heat-resistance, they can be utilized to promote chemical reactions in their nanospaces.
Previously, Professor Onoe and his group from Nagoya University found that 1D uneven structured (peanut-shaped) C60 polymer with metallic nature was formed by electron-beam (EB) irradiation of a pristine C60 film under ultrahigh vacuum (UHV) conditions. This film has sub-nm spaces and exhibits a high electric conductivity and high heat-resistance as an organic film, so it is expected that the sub-nm space can act as a specific reaction field.
In this study, the researchers formed 1D peanut-shaped C60 polymer film from EB-irradiation of a pristine C60 film, air-exposed 1D C60 polymer film at RT, and compared the change in IR spectra of the film before and after atmospheric-air exposure under UHV conditions. The CO32− anion was formed by the reaction between CO2 and H2O in the sub-nm space of the 1D peanut-shaped C60 polymer film at RT. However, as for a pristine C60 film that had a similar sub-nm space inside, no significant differences in their spectral features before and after atmospheric-air exposure were observed.
The researchers found that the 1D metallic peanut-shaped C60 polymer as a framework of the sub-nm space also played a crucial role in promoting the reaction at RT. CO2 and H2O reacted in the sub-nm space of the 1D polymer film, forming H2CO3, which was decomposed and produced 2H+ + CO32−.
First-principles calculations revealed that the CO2 molecule is stacked as a bridge between the concave portions of adjacent 1D C60 polymer frameworks via locally induced Coulomb interactions. Such induced charge polarization activates CO2 both by weakening its double-bonds and by lowering its lowest unoccupied molecular orbital energy due to the bending motion. This suggests that the bending motion promotes the CO2 + H2O reaction from the obtuse-angle side, resulting in reduction of the activation energy.
The reaction proceeded in the 1D peanut-shaped C60 polymer film at RT, which means that the sub-nm space of C60 film not only plays a role in the immobilization of CO2, but also acts as a specific reaction field that has the potential to turn CO2 into valuable products. Hence, the 1D metallic peanut-shaped C60 polymer as a framework of the sub-nm space also plays a crucial role in promoting the reaction at RT. This group’s discovery is significant in terms of academics and achieving Sustainable Development Goals (SDGs).
Figure 1
Figure 2
Figure 3
Figure 4
The article, “Immobilization of CO2 at Room Temperature Using the Specific Sub-NM Space of 1D Uneven-Structured C60 Polymer Film,” was published in Advanced Sustainable Systems at https://doi.org/10.1002/adsu.202000156.
