RF resins under acidic conditions can be used for the photocatalytic generation of H2O2
A group of researchers from the Osaka University Research Center for Solar Energy Chemistry synthesized resorcinol–formaldehyde (RF) resins, photocatalysts that can generate hydrogen peroxide (H2O2) from water and dioxygen under sunlight illumination conditions.
Since H2O2 used as bleach and disinfectant is also used as fuel in a fuel cell, it is drawing attention as an energy carrier that stores and transports recyclable energy. Conventional H2O2 production required multi-step operations with high energy consumption in order to react H2 and O2, whereas photocatalytic reaction for producing H2O2 from water and O2 using solar energy was at the center of attention. However, it was difficult to perform four-electron oxidation of water and selective two-electron reduction of O2 by common photocatalysis, so the development of new photocatalysts was sought after.
In 2019, this group discovered that resorcinol–formaldehyde (RF) resins, which had been employed as “insulator” polymers, served as semiconductor photocatalysts by simple high-temperature hydrothermal synthesis. This catalyst produces H2O2 at more than 0.5% solar chemical converter (SCC) efficiency, the highest efficiency of powder photocatalysts.
In this study, the group paid attention to the pH of a solution for the high-temperature hydrothermal synthesis process. Generally, RF resins are synthesized in a reaction between resorcinol and formaldehyde in basic ~ neutral conditions. The researchers synthesized resins by adjusting the pH of a solution during the synthesis process and found that RF resins prepared by the acid-catalyzed high-temperature hydrothermal method at pH levels lower than 4 exhibited enhanced photocatalytic activity for H2O2 generation.
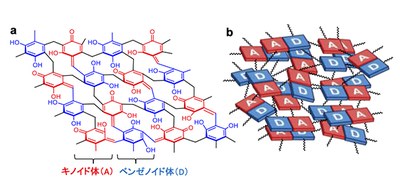
Under acidic conditions, lower-degree-crosslinked resins are formed, wherein the donor–acceptor (D–A) units exhibit a stronger π-stacking because of the structural flexibility. The stronger π-stacking in the RF-acid resins enhances the delocalization of the π-electrons and creates the resins with lower bandgap of 1.7eV under irradiation with near-infrared (λ < 720 nm) light, improving the conductivity.
The irradiation of the RF-acid resins with simulated sunlight in water with atmospheric-pressure O2 generates H2O2 at a solar-to-chemical conversion efficiency of 0.7%. Inorganic / organic acids, such as sulfuric acid (H2SO4), hydrogen chloride (HCl), nitric acid (HNO3), oxalic acid (C2H2O4), and acetic acid (CH3COOH), can be used as acid solutions. In addition, since the prepared photocatalysts are spherical particles of 3~5μm in diameter and easy to handle, they can be used for various applications.
This RF resin can be prepared by the acid-catalyzed high-temperature hydrothermal method (~523 K) using common acids at pH < 4. The photocatalytic generation of hydrogen peroxide from water and dioxygen under sunlight is a promising strategy for the artificial photosynthesis of a liquid fuel. By applying this photocatalyst design, it will become possible to create H2O2 synthesis catalysts with high activity.
Figure 1
Figure 2
Figure 3
The article, “Solar-to-hydrogen peroxide energy conversion on resorcinol–formaldehyde resin photocatalysts prepared by acid-catalysed polycondensation,” was published in Communications Chemistry at DOI: https://www.nature.com/articles/s42004-020-00421-x.
