Photocatalyst for making ammonia from sun, air, and water at normal temperature and pressure
A research group from the Research Center for Solar Energy Chemistry of Osaka University has demonstrated that ultraviolet light irradiation of a semiconductor in water containing chloride anions (Cl–) under nitrogen gas (N2) flow efficiently produces ammonia (NH3).
NH3 is a major component in fertilizer and attracts much attention as a promising carbon-free energy carrier. Conventional ammonia synthesis is conducted under very high pressure and temperature; however, in principle, it is possible to produce ammonia from water and nitrogen gas using solar energy through photocatalyst reaction, which is anticipated as an energy saving technology.
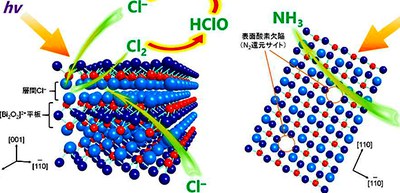
In this study, the group developed a semiconductor, bismuth oxychloride with surface oxygen vacancies (BiOCl-OVs). The researchers demonstrated that sunlight irradiation of powder photocatalysts suspended in water containing chloride anions (Cl–) such as seawater under N2 flow efficiently produced ammonia. This photocatalyst technique generates NH3 with 0.05% solar-to-chemical conversion efficiency, which is equivalent to natural synthesis by plants (~0.1%).
This catalyst absorbs ultraviolet light and undergoes photodecomposition to O2 and Cl–, producing valence band holes (VB h+) for water oxidation and conduction band electrons (CB e–) for N2 reduction. The surface OVs behave as the N2 reduction sites. On the other hand, the VB h+ are consumed by self-oxidation of interlayer Cl–, producing Cl2. Since this reaction proceeds more rapidly than water oxidation, the photocatalytic sequence proceeds efficiently, promoting efficient NH3 production. The Cl2 photoformed in water is in equilibrium with hypochloric acid (HClO). The hypochloric acid (HClO) formed absorbs ultraviolet light and undergoes photodecomposition into O2 and Cl–.
These consecutive photoreactions produce NH3 with water as the electron donor. The Cl– in solution compensates for the removed interlayer Cl– and prevents subsequent reactions, thus suppressing catalyst deactivation.
Solar to Chemical Conversion (SCC) has been studied for quite some time, but slow water oxidation has prevented highly efficient SCC. The researchers demonstrated that the combination of (1) Oxidization of interlayer Cl- by valence band holes, (2) Photodecomposition of HCIO, and (3) Cl-compensation stably produces NH3.
Metal oxide semiconductors can be used for organic synthesis. By applying this group’s technique, it will become possible to produce catalysts for ammonia photosynthesis and artificial photosynthesis systems using seawater, an abundant resource.
Figure 1
Figure 2
The article, “Photocatalytic Dinitrogen Fixation with Water on Bismuth Oxychloride in Chloride Solutions for Solar-to-Chemical Energy Conversion” was published in Journal of the American Chemical Society at DOI: https://doi.org/10.1021/jacs.0c01683
