Producing H2O2 with sunlight, water, and oxygen by unique photocatalysts
A group of researchers from the Osaka University Research Center for Solar Energy Chemistry developed resorcinol–formaldehyde (RF) resins, photocatalysts for generating hydrogen peroxide (H2O2) from water and dioxygen under sunlight illumination conditions.
H2O2, used as bleach and disinfectant, is also used as fuel for fuel batteries as oxidizing and reductant agents, drawing attention as an energy carrier that stores and transports recyclable energy as well.
Conventional H2O2 production requires multi-step operations with high energy consumption in order to react H2 and O2, whereas photocatalytic reaction to produce H2O2 from water and O2 using solar energy is anticipated as an energy-saving process. However, it was difficult to perform four-electron oxidation of water and selective two-electron reduction of O2 by common photocatalysis. Thus, the development of new photocatalysts had been sought after.
RF resins, synthetic polymers used for coatings and adhesives, are insulators and had not been regarded as candidates for semiconductor photocatalysts. However, this group developed RF photocatalysts to produce H2O2 from water and O2 under sunlight illumination using their unique synthesis method that employs high-temperature hydrothermal synthesis, finding that RF resin powders prepared by simple high-temperature hydrothermal synthesis behaved as semiconductor photocatalysts, a world first.
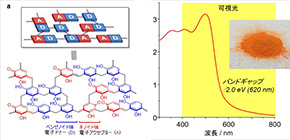
The researchers developed photocatalysts through hydrothermal synthesis of RF resins at high temperature (over 200℃), which is higher than that of common synthesis (~100℃). The catalysts absorb broad-wavelength light up to 700 nm, producing H2O2 at more than 0.5% solar chemical converter (SCC) efficiency, which is higher than the average solar-to-biomass conversion efficiency of natural photosynthesis by typical plants (~0.1%). Efficiency of more than 0.5% SCC is higher than that of any other powder catalysts used in artificial photosynthesis that has been reported so far.
This group also clarified the causes of particularly high activity of RF resins for H2O2 generation by high-temperature hydrothermal synthesis. That is, high-temperature hydrothermal synthesis produces low-bandgap RF resins comprising π-conjugated and π-stacked benzenoid–quinoid donor–acceptor (D-A) couples. In RF resins, the photogenerated valence band holes oxidize water, producing O2, and the conduction band electrons (CB e-) promote two-electron reduction of O2, producing H2O2. In addition, the catalyst is less active for subsequent H2O2 decomposition. Because of these characteristics, RF resins exhibited very high H2O2 generation.
The developed photocatalyst resins are spherical particles with an average diameter of ~1µm. It is easy to handle them, so they can be put to practical use through various processing steps. In addition, by applying this photocatalyst design, it will become possible to produce more active H2O2 synthesis catalysts.
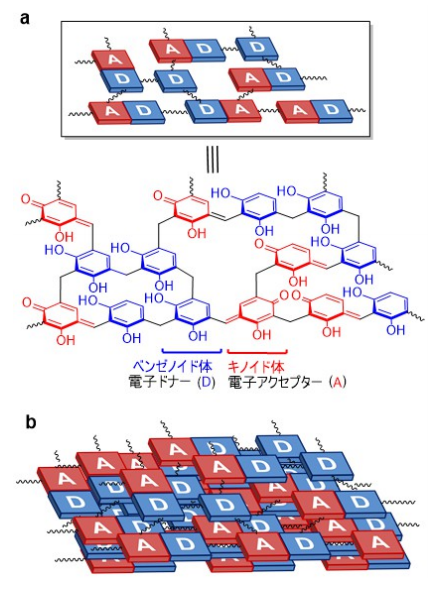
Figure 1

Figure 2
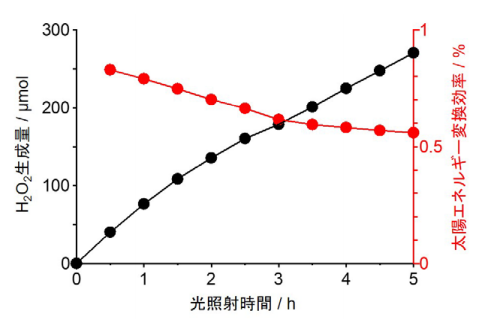
Figure 3
The article, “Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-tohydrogen peroxide energy conversion,” was published in Nature Materials at DOI: https://doi.org/10.1038/s42003-019-0564-6 .
Related links
