
LED-ing the way: a clean and convenient method to oxidize plastic surfaces for industry
Osaka University researchers successfully oxidize the surface of polypropylene using radical chemistry, in a non-polluting, LED-light-driven reaction with prospective uses in the plastics industry
Polypropylene (PP) is everywhere, being one of the most widely used plastics in human life. A versatile material, its naturally inert surface can be modified for specific applications. Researchers at Osaka University have now developed a convenient light-driven process for oxidizing PP without harmful waste.
As reported in ChemComm , the process uses radicals to make the plastic react. The surface of PP bristles with methyl groups (–CH 3 ), which constitute the side chains of the polymer. The strong C–H bonds in methyl groups make PP an unreactive material, which for many purposes is exactly what is needed. However, these bonds can be cleaved by the highly reactive chlorine dioxide radical, ClO 2 • .
“In applications like printing and medical materials, plastics must be surface-modified,” explains study co-author Tsuyoshi Inoue. “Oxidizing C–H bonds is a textbook case in organic chemistry. With polymers, however, the risk is that anything strong enough to do this may also break the C–C bonds of the main chain, ripping the polymer apart. Luckily, the ClO 2 • radical is selective to react the side chain.”
The highly reactive radical is easily made by mixing sodium chlorite and hydrochloric acid. It then just needs to be photochemically activated—for this, the Osaka team chose an LED lamp as the light source. The activated ClO 2 • now splits into Cl • , which whips off an H atom from the side chain of PP; and O 2 , which marches in afterward to oxidize the exposed –CH 2 • group.
As a result, while the bulk polymer remains intact, the surface now bears a multitude of carboxylic acid groups (–CO 2 H), with major effects on the chemical reactivity. For example, the colorless plastic can now be stained with cationic dyes, such as Rhodamine B or Brilliant Green, which react with the anionic carboxylate ions. The originally water-repellent surface also becomes more hydrophilic.
“The reaction actually proved to be doubly selective for our purposes,” says lead author Kei Ohkubo. “Not only did it cleave the C–H instead of C–C bonds, it specifically oxidized those on the side chain, even though they are stronger than those on the main chain. This is because the oxidation step, involving O 2 , is most favorable when the target for oxidation is CH 2 • .”
Previous methods for oxidizing olefinic polymers such as PP and polyethylene were either poorly controlled or highly polluting. The new process is thus the first clean and convenient solution to this problem, and may prove to be a valuable industrial tool in the customization of synthetic plastics.
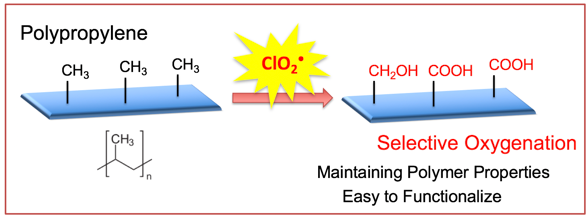
Fig. 1: Surface oxygenation of side-chain methyl groups in polypropylene under photoirradiation with chlorine dioxide.
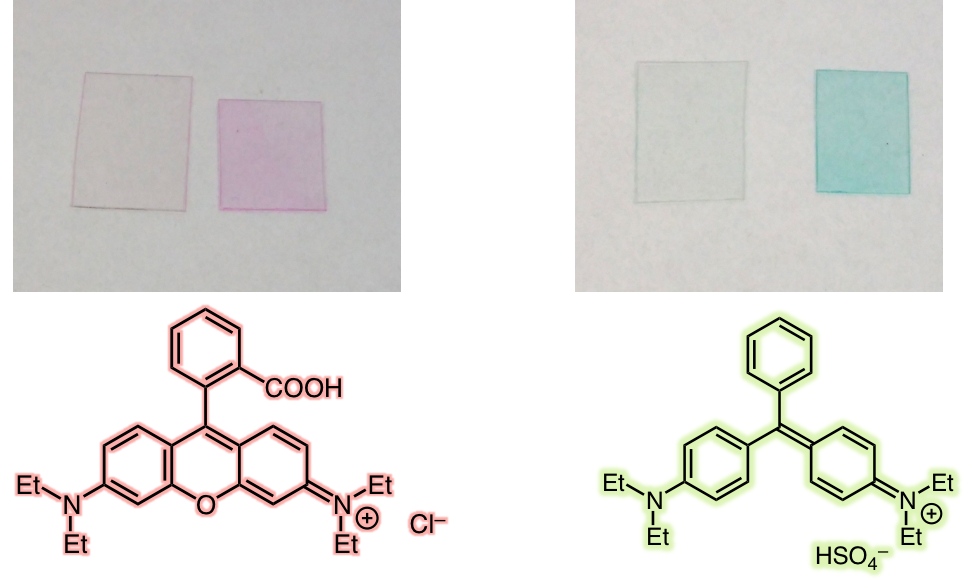
Fig. 2: PP films after water-soluble ink treatments without/with ClO 2 photooxygenation.
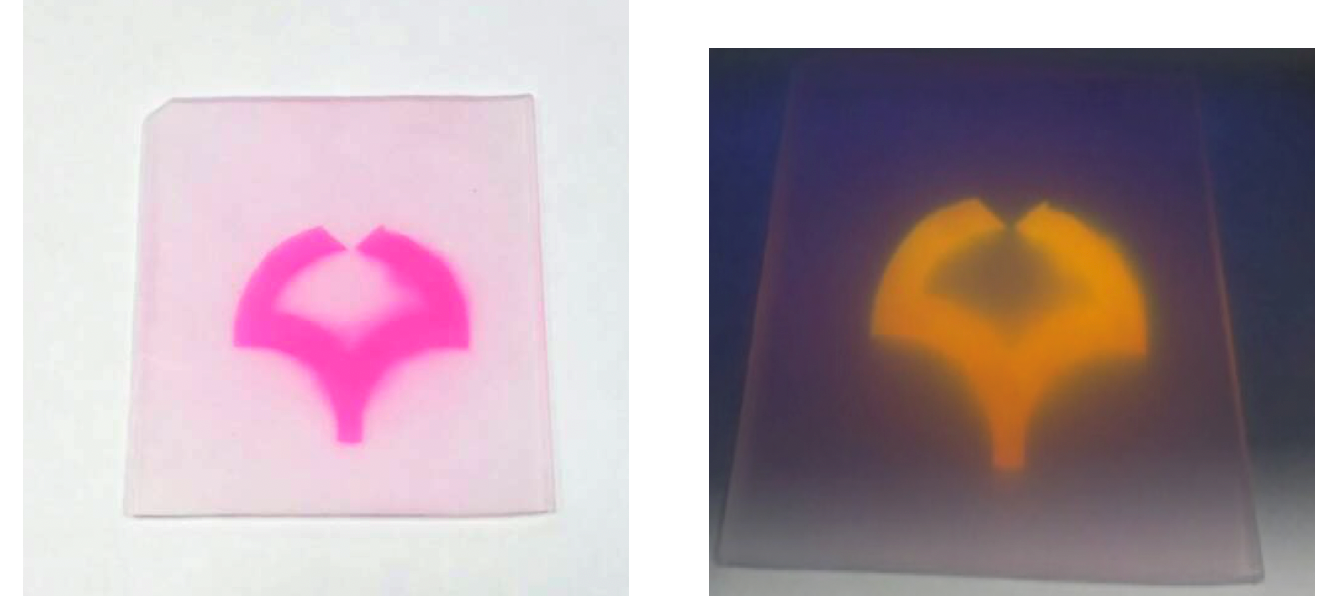
Fig. 3: Spot staining after treatment with rhodamine as a red ink after site-selective photooxygenation. Spot emission under black-light irradiation.
The article, “Photochemical C–H oxygenation of side-chain methyl groups in polypropylene with chlorine dioxide,” was published in ChemComm at DOI: https://doi.org/10.1039/c9cc01037h .
Related links
