Functions of gene causing brain abnormality clarified
Development of autophagy-targeting therapeutic methods for preventing cancer and lifestyle-related diseases
Autophagy is a degradation system in eukaryotic cells to perform intracellular cleansing. A membrane structure called an autophagosome is formed, which travels through the cytoplasm to a lysosome. Following autophagosome–lysosome fusion, the contents of the autophagosome are degraded within the lysosome.
Patients with Joubert syndrome manifesting mental, motor, and developmental delay due to brain abnormality had been found to have inositol polyphosphate-5-phosphatase E (INPP5E) mutations, but it was not clear how they were involved in the development of the disease.
A group of researchers led by Professor YOSHIMORI Tamotsu at the Laboratory of Intracellular Membrane Dynamics, Graduate School of Frontier Biosciences, Osaka University, clarified that INPP5E, a gene causing the congenital disorder Joubert syndrome, which manifests brain abnormality, controlled autophagy, a world first.
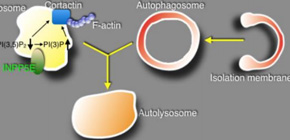
This group clarified that INPP5E promoted autophagosome-lysosome fusion, the last step of autophagy, by turning phosphoinositide PI(3,5)P 2 , which is contained in the lysosome membranes, into PI(3)P. This group also demonstrated that a decrease in PI(3,5)P 2 and an increase in PI(3)P induced the activation of cortactin, a protein that promotes actin polymerization, and that consequently actin filaments were formed on the surface of lysosome, contributing to the autophagosome-lysosome fusion.
From this, the possibility that compromised autophagy in neuronal cells might cause Joubert syndrome was suggested. It was also clarified that INPP5E mutations found in patients with Joubert syndrome inhibited autophagy. These results suggest that the development of Joubert syndrome was caused by compromised autophagy.
This group’s research results clarified not only a mechanism behind the development of Joubert syndrome, but also a part of the control system of autophagy, which serves as an inhibitor of various important disorders such as cancer, neurodegenerative disorder, inflammatory disorder, and lifestyle-related diseases. It is hoped that this group’s achievement will be used for the development of preventive care strategies targeting autophagy.
Abstract
Autophagy is a multistep membrane traffic pathway. In contrast to autophagosome formation, the mechanisms underlying autophagosome–lysosome fusion remain largely unknown. Here, we describe a novel autophagy regulator, inositol polyphosphate-5-phosphatase E (INPP5E), involved in autophagosome–lysosome fusion process. In neuronal cells, INPP5E knockdown strongly inhibited autophagy by impairing the fusion step. A fraction of INPP5E is localized to lysosomes, and its membrane anchoring and enzymatic activity are necessary for autophagy. INPP5E decreases lysosomal phosphatidylinositol 3,5-bisphosphate (PI(3,5)P 2 ), one of the substrates of the phosphatase, that counteracts cortactin-mediated actin filament stabilization on lysosomes. Lysosomes require actin filaments on their surface for fusing with autophagosomes. INPP5E is one of the genes responsible for Joubert syndrome, a rare brain abnormality, and mutations found in patients with this disease caused defects in autophagy. Taken together, our data reveal a novel role of phosphoinositide on lysosomes and an association between autophagy and neuronal disease.
To learn more about this research, please view the full research report entitled “ Autophagosome-lysosome fusion in neurons requires INPP5E, a protein associated with Joubert syndrome ” at this page of the EMBO Journal website.
Related link
