New Catalytic Function of Organomagnesium Compounds Clarified
From right under our noses: a new generation of metallic compounds from all around us
A group of researchers led by MASHIMA Kazushi (Professor) and TSURUGI Hayato (Associate Professor) at the Graduate School of Engineering Science, Osaka University, designed organometallic compounds at a molecular level by making use of magnesium and achieved catalytic isomerization reaction of hydrocarbons through C-H bond activation. This is a new type of catalytic action of organomagnesium compounds. The organomagnesium compounds developed by this group are easily available at a low price and have low toxicity. This group's achievement will possibly lead to the development of organic semiconductor materials to be used for organic EL displays.
Abstract
Organomagnesium complexes were synthesized from N,N-dialkylamineimine ligands and dibenzylmagnesium by benzylation of the imine moiety. 3-Aryl-1-propynes reacted with the magnesium complex to form the corresponding tetraalkynyl complexes, which acted as catalysts for the transformation of these terminal alkynes into allenes and further to internal alkynes under mild conditions. To the best of our knowledge, this example is the first of an organomagnesium-catalyzed isomerization of alkynes. Notably, the reactions proceeded through temporally separated autotandem catalysis, thus allowing the isolation of the allene or internal alkyne species in good yields. Mechanistic experiments suggested that the catalytically active tetraalkynyl complexes consist of a tautomeric mixture of alkynyl-, allenyl-, and propargylmagnesium species.
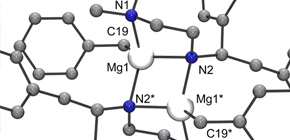
Synthesis and Molecular Structure of Organomagnesium Catalyst 1 .
Figure 1. ORTEP Drawing of Organomagnesium Catalyst 1 .
Figure 2. Isomerization of Terminal Alkynes via C-H Bond Activation
Figure 3. Reaction of Organomagnesium compound 1 with phenylacetylene
To learn more about this research, please view the full research report entitled " Organomagnesium-Catalyzed Isomerization of Terminal Alkynes to Allenes and Internal Alkynes " at this page of the Chemisty - A European Journal website.
Related Link
