Smallest Nucleic Acid Medicine Delivery System Developed
An innovation in cancer treatment methods
A group of researchers led by YAMAMOTO Hirofumi ( Associate Professor, Department of Gastroenterological Surgery, Department of Surgery, Graduate School of Medicine, Osaka University) and Xin Wu (4th year doctoral program student, Department of Gastroenterological Surgery, Department of Surgery, Graduate School of Medicine, Osaka University) succeeded in making the world's smallest intravenously injectable super apatite gene delivery reagents at just 10nm, while also succeeding in accumulating nucleic acids in solid tumors with considerable efficiency.
Abstract
RNA interference (RNAi) technology is currently being tested in clinical trials for a limited number of diseases, including eye, skin, liver and kidney. However, systemic delivery of small interfering RNA (siRNA) to solid tumors has not yet been achieved in clinics. We introduce an in vivo pH-sensitive delivery system for siRNA using sonicated carbonate apatite (CA) nanoparticles, which is the smallest class of nanocarrier, sized 10nm in majority. These carriers consist simply of inorganic ions CO 3 2- , Ca 2+ , and PO 4 3- , and accumulate specifically in tumors, yet they cause no serious adverse events in mice and Cynomolgus monkeys. Intravenously administered CA-siRNA abundantly accumulated in the cytoplasm of tumor cells at 4 h, indicating quick achievement of endosomal escape. CA-survivin-siRNA induced apoptosis in HT29 tumors and significantly inhibited in vivo tumor growth of HCT116, to a greater extent than two other in vivo delivery reagents, liposome and atelocollagen.
Main novelty
RNAi technology for solid tumors, especially in systemic administration, still faces numerous hurdles. In addition to stability in the blood flow, an ideal system requires (i) quick delivery of siRNA to the tumor cells since siRNA easily gets damage, ii) sufficient cellular uptake, iii) radical endosomal escape, permitting a rapid cytoplasm diffusion. The CA system surmounted every hurdle. Owing to its distinctly innovative delivery efficiency and anti-tumor activity in vivo, we designated the sonicated small nanoparticle as super carbonate apatite. This simple, inexpensive, and highly efficient in vivo delivery system may open a new avenue in nucleotide medicine.
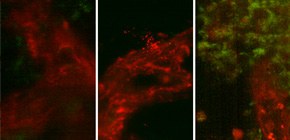
Figure: In a mouse subcutaneous tumor, vessels were marked in red, and nucleotides were labeled in green. With the super apatite, much more nucleotides were accumulated in tumor cells as compared to the other systemic delivery reagents.
To learn more about this research, please view the full research report entitled "Innovative Delivery of siRNA to Solid Tumors by Super Carbonate Apatite" at this page of the PLOS ONE website.
Related Link
