Basic properties of amyloid fibrils causing Parkinson's disease clarified
Amyloid fibrils transition states depend on temperature
A group of researchers (at Eötvös Loránd University, Hungary, and at Tottori University, Japan) led by GOTO Yuji (Professor, Institute for Protein Research, Osaka University) have discovered that amyloid fibrils causing Parkinson's disease denatured completely at low temperature.
Denatured proteins are unstable and generate aggregates through intermolecular interaction. Denatured and misfolded proteins form amyloid fibrils and cause amyloid diseases such as Alzheimer's disease and Parkinson's disease; however, why amyloid fibrils are formed and details re the stability of the fibrous structures formed have been unknown.
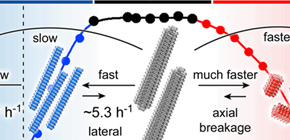
Globular proteins denature when temperature changes from internal body temperature to extremely high or extremely low temperature. Amyloid fibrils are formed through heat denaturation and cold denaturation. In amyloid fibrils, cold denaturation occurred at the mild temperature of 20°C and brought about full cold denaturation at 0°C.
This group, examined how the structure of amyloid fibrils changed in temperatures ranging from 0 to 110°C. In experiments using 6 types of amyloid fibrillogenesis proteins including alpha-synuclein protein (αSN), all amyloid fibrils irreversibly depolymerized at temperatures over 60°C (heat-denatured to monomers). Heat denaturation at high temperature doesn't vary with the kind of protein and was a common character in amyloid fibrils. At low temperatures of 20 °C or lower, amyloid fibrils alone cold-denatured at low temperature and were reversibly depolymerized. From these findings, it has been suggested that a decrease in stability of fibrous structure at low temperature is dependent on the amino acid sequence of proteins.
Furthermore, in order to identify factors in cold denaturation of αSN fibrils, this group compared cases of variant proteins with measured responses and found that factors in cold denaturation of αSN fibrils were in burial of charged residue in fibrous structure. From these experiments, this group clarified how cold denaturation occurs in amyloid fibrils, which differs from that of globular proteins.
This group of researchers demonstrated that amyloid fibrils transition states depend on temperature and assessed the degree of conformational stability. This group's achievements suggested that homeostasis of αSN fibrils was higher than that of other fibrils and so it was possible to reverse the changes. Understanding of causes of instability and mechanisms for decreasing the stability of fibrils will lead to an understanding of the formation of fibrils and the development of methods for their disaggregation and clearance and will thereby assist in the development of treatment and prevention of diseases, such as Parkinson's, caused by amyloid fibrils.
Abstract
Although amyloid fibrils are associated with numerous pathologies, their conformational stability remains largely unclear. Herein, we probe the thermal stability of various amyloid fibrils. Α-Synuclein fibrils cold-denatured to monomers at 0–20 °C and heat-denatured at 60–110 °C. Meanwhile, thefibrils of β2-microglobulin, Alzheimer's Αβ1-40/Αβ1-42 peptides, and insulin exhibited only heat denaturation, although they showed a decrease in stability at low temperature. A comparison of structural parameters with positive enthalpy and heat capacity changes which showed opposite signs to protein folding suggested that the burial of charged residues in fibril cores contributed to the cold denaturation of α-synuclein fibrils. We propose that although cold-denaturation is common to both native proteins and misfolded fibrillar states, the main-chain dominated amyloid structures may explain amyloid-specific cold denaturation arising from the unfavorable burial of charged side-chains in fibril cores.
Figure 1
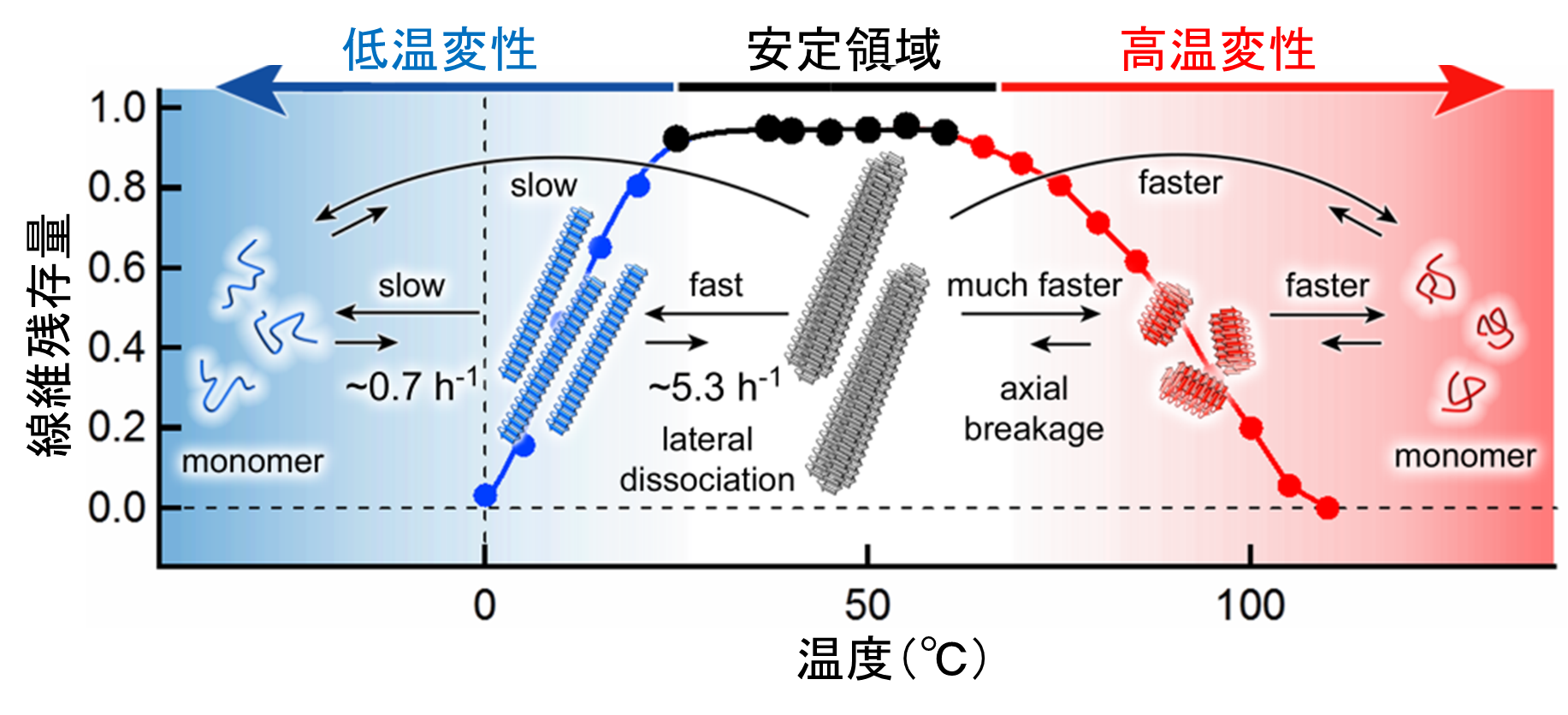
Figure 2

Figure 3

Figure 4
To learn more about this research, please read the full research report entitled " Cold Denaturation of α-Synuclein Amyloid Fibrils " at this page of Angewante Chemie website.
Related links :
