Clarification and control of the mechanism of handedness selectivity in hydrogen-bonding 2-helical networks
Under the leadership of MIYATA Mikiji , Visiting Professor, Institute of Scientific and Industrial Research, Osaka University, TOHNAI Norimitsu , Associate Professor, HISAKI Ichiro , Assistant Professor, SASAKI Toshiyuki , student in the second-term of the 3rd year of a doctoral program, Graduate School of Engineering, Osaka University, SATO Hisako , Professor, Graduate School of Science, Ehime University, and TUZUKI Seiji , Chief Fellow, Advanced Industrial Science and Technology (AIST), a group of researchers has clarified the mechanism for handedness selectivity in hydrogen-bonding 2-helical networks by conducting single crystal X-ray structure analyses of organic salts composed of chiral amino and achiral carboxylic groups.
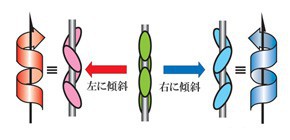
Chirality, a property of asymmetry in molecules, is very important in developing new drugs and configuration technology for the handedness (dextrorotatory and levorotatory) is essential in additional applications. Not only chirality of single molecules, but also supramolecular chirality, the non-symmetric arrangement of molecular components in a non-covalent assembly, has attracted attention. In many cases, supramolecular chirality depends on molecular chirality although opposite-handed supramolecular assemblies are also obtained. Thus, handedness control in crystalline state is difficult. Using a method of supramolecular tilt chirality in organic salts composed of chiral amino and achiral carboxylic acid, this group succeeded in differentiating right and left in 2 helical assemblies without changing amino chirality.
This group's achievement demonstrated that chirality of helical assemblies can be easily controlled even if molecules with the same chirality are used, expanding the possibility of natural product-derived molecules with only one-handedness as well.
This method to control right and left helicity can be used for the development of drugs and medicines and circular polarized light obtained from luminescent molecules with chirality is also expected to be used in the development of 3D displays.
Abstract
Chiral molecules preferentially form one-handed supramolecular assemblies that reflect the absolute configuration of the molecules. Under specific conditions, however, the opposite-handed supramolecular assemblies are also obtained because of flexibility in the bond length and reversibility of non-covalent interactions. The mechanism of the handedness selectivity or switching phenomenon remains ambiguous, and most phenomena are observed by chance. Here we demonstrate the construction of chiral hydrogen-bonded twofold helical assemblies with controlled handedness in the crystalline state based on crystallographic studies. Detailed investigation of the obtained crystal structures enabled us to clarify the mechanism, and the handedness of the supramolecular chirality was successfully controlled by exploiting achiral factors. This study clearly reveals a connection between molecular chirality and supramolecular chirality in the crystalline state.
Figure 1
Figure 2
To learn more about this research, please read the full research report entitled " Linkage Control between Molecular and Supramolecular Chirality in 21-Helical Hydrogen-bonded Networks Using Achiral Components " at this page of the Nature Communications website.
Related links :


