New Breakthrough in Treatment for Rare Skin Condition
Drug to treat skin lesions in tuberous sclerosis complex approved, a world first
Scientists at Osaka University developed a new drug treating skin lesions in tuberous sclerosis complex (TSC), a rare intractable disease, a world first. Following the physician-led clinical studies I and II by Osaka University, Phase III clinical trials by a pharmaceutical company, the drug was approved within 6 months of the drug application by the SAKIGAKE Designation System, an early approval system led by the Japanese government, on March 23, 2018 and was then commercialized.
This drug is the first drug designated by the SAKIGAKE system. The details of physician-led clinical studies I and II were published in JAMA Dermatology in January 2017.
TSC is an inherited autosomal dominant disease caused by mutations in TSC1 (hamartin) and TSC2 (tuberin) genes. Mutations in the TSC1 and TSC2 tumor suppressor genes cause hyperactivation of the mTORC1 (mammalian target of rapamycin complex 1), developing neoplastic lesions called hamartoma in numerous organs, such as the brain, skin, kidneys, and lungs.
The symptoms of TSC include epilepsy, learning disabilities, developmental delays, and autism spectrum disorder in addition to hamartoma. Of the various symptoms, skin problems, such as facial angiofibromas, develop in more than 90 percent of patients with TSC. Hemorrhage, secondary bacterial infection, pain, and functional disorder associated with lesions inflict suffering on patients, severely compromising their quality of life for cosmetic reasons. However, currently, the established therapeutics are limited to surgical therapies, which are difficult to perform on small children and patients with intellectual disabilities without general anesthesia.
The systemic administration of sirolimus (Rapamycin) reduces the tumor volume and lessen the redness of tumors in TSC; however, the systemic treatment may cause adverse effects. Thus, the research group led by Mari Wataya-Kaneda proceeded with research and development of a topical gel of mTORC1 inhibitors as a safer therapeutic agent, considering topical sirolimus formulations in order to enhance the absorption of effective ingredients with large molecular weight.
This topical sirolimus gel is the only drug which can be locally applied to skin lesions in TSC. The approval of this painless, safe, and easy-to-use drug has enabled safe and effective treatment for skin lesions in TSC. Also, the SAKIGAKE system and industry-government-university collaboration worked extremely well in the approval of the drug, which will be helpful in developing drugs for treating other intractable diseases in the future.
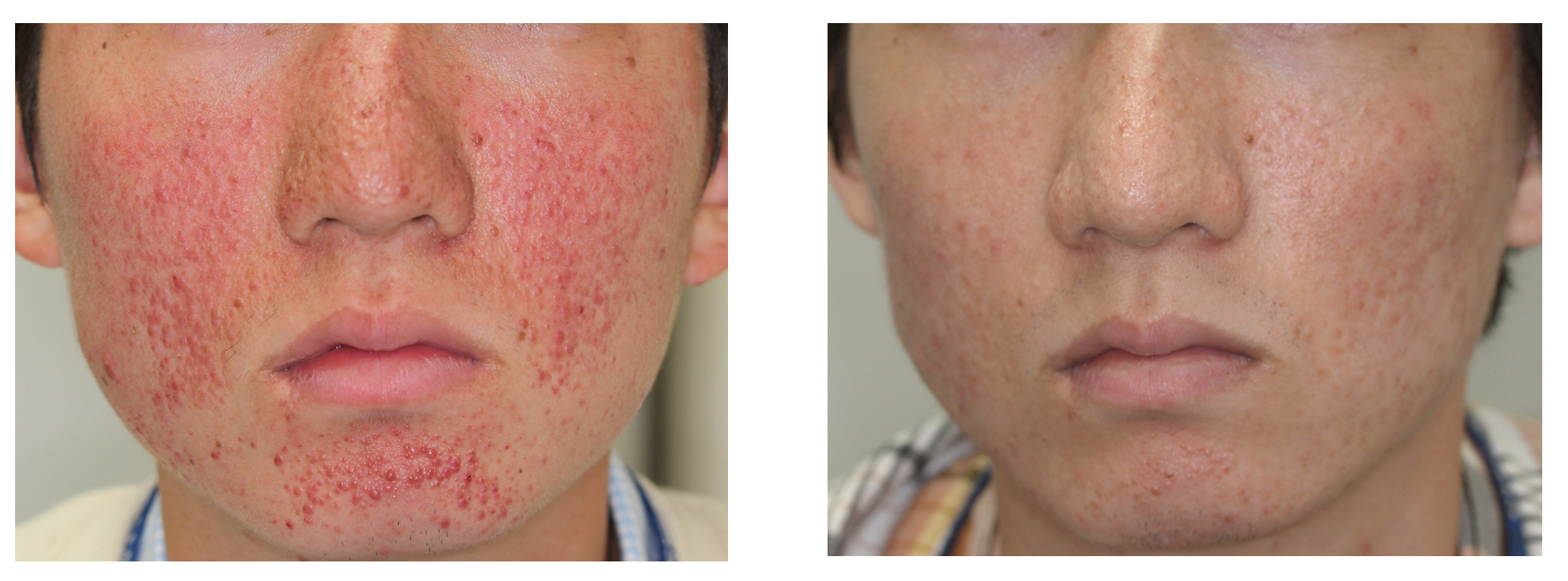
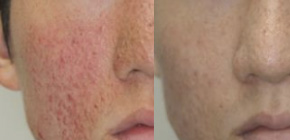
Fig.1 Photographs of Facial Angiofibromas in TSC Before and After Treatment with Sirolimus gel.
(credit: Osaka University)
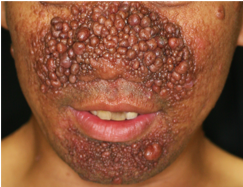
Fig.2. Characteristic Serious Facial Angiofibromas in TSC.
(credit: Osaka University)
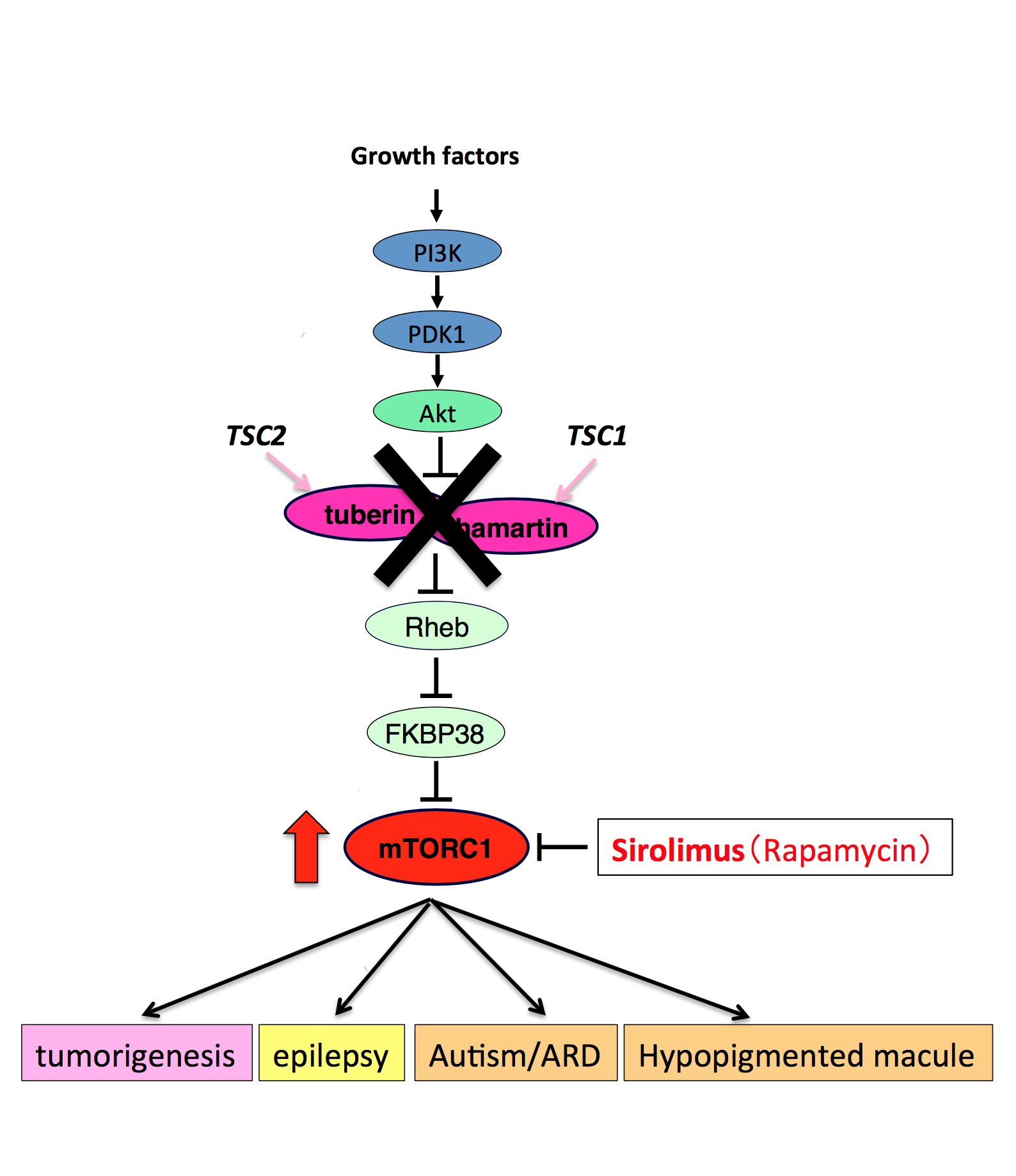
Fig.3. Pathophysiology of Tubrerous Sclerosis Complex
(credit: Osaka University)
To learn more about this research, please view the full research report entitled "Efficacy and Safety of Topical Sirolimus Therapy for Facial Angiofibromas in the Tuberous Sclerosis Complex A Randomized Clinical Trial" at this page of JAMA Dermatology.
Related links
