Short step, two-thirds of conventional methods, synthesis of inexpensive liquid crystal compound achieved
Discovery is expected to promote development of new low-cost functional materials
A group of researchers affiliated with Osaka University-Daikin Industries (Fluorine Chemistry) Joint Research Course developed a new reaction for developing a liquid crystal compound possessing a tetrafluoroethylene-bridging structure in a short-step process.
Researchers at Osaka University's Graduate School of Engineering:
• OGOSHI Sensuke (Professor)
• OHASHI Masato (Lecturer)
Liquid crystal compounds possessing tetrafluoroethylene-bridging structures are sought after as materials for next-generation liquid displays made from tetrafluoroethylene, fluorine-based resin and key industrial materials.
Tetrafluoroethylenes is, industrially, a very inexpensive chemical compound with many applications. One product employing tetrafluoroethylene is known by the trade name Teflon® and used as a non-stick coating agent in a variety of applications. However, applications of tetrafluoroethylene have been limited to the production of a fluorine-based resin and have never been used as a manufacturing material in a broad range of chemical products such as medicines or agricultural chemicals.
On the other hand, a liquid crystal compound bearing a tetrafluoroethylene-bridging structure has been sought after as a material for next-generation liquid crystal displays. If a method of synthesizing such a liquid crystal compound in large quantities at a low price can be developed, it will give momentum to the practical use and diffusion of high-performance optical devices. However, typical synthesis methods of chemical compounds lack versatility and cost a lot or use highly toxic fluorinating agents for the synthesis, so environmentally friendly and inexpensive synthesis methods have been sought.
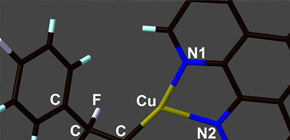
This group of researchers worked on the development of a reaction which would connect different aromatic rings to each of two carbon atoms in a tetrafluoroethylene molecule. Specifically, this group found that fluoroalkyl copper complexes were obtained in good yield via the methods of Carbometalation and Carbocupration.
This group has clarified that, in the obtained fluoroalkyl copper complexes, it was possible to replace the copper atom with the second aromatic ring via a reaction with iodobenzene. Chemical compounds into which different two aromatic rings were introduced can be converted to liquid compounds possessing tetrafluoroethylene-bridging structures in good yields in a short-step process. This group's discovery is expected to further promote the development of new fluoline-containing functional materials at a low cost and the practical use and dissemination of high-performance optical devices.
Abstract
We report a copper-mediated synthesis of a variety of 1,2-difunctionalized-1,1,2,2-tetrafluoroethylene derivatives via the carbocupration of tetrafluoroethylene. The key synthetic intermediates, 2-aryl-1,1,2,2-tetrafluoroethylcopper complexes, can be easily prepared, stored, and used as fluoroalkylation reagents. The molecular structure was unambiguously determined by X-ray crystallography and NMR analysis. We applied this method to the short-step synthesis of a liquid-crystalline compound bearing a tetrafluoroethylene-bridging structure.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
To learn more about this research, please view the full research report entitled " Fluoroalkylcopper(I) Complexes Generated by the Carbocupration of Tetrafluoroethylene : Construction of a Tetrafluoroethylene-Bridging Structure" at this page of the Journal of the American Chemical Society website.
Related link :
• Ogoshi Research Group , Division of Applied Chemistry, Graduate School of Engineering
