Approaching an Ideal Amino Acid Synthesis Using Hydrogen
Researchers from Osaka University develop a green and sustainable method for reductive alkylation of multiply substituted amines
Amines are an essential part of our everyday lives; a fact supported by the number of bioactive molecules, including natural products, pharmaceuticals, and agrochemicals that contain amine motifs. Therefore, the development of green, sustainable, and waste-minimized approaches for the synthesis of amines and amino acids, using readily available catalysts and less toxic reagents, remains a significant challenge.
A group of researchers from Osaka University has now developed a practical and environmentally innocuous method for the functionalization of multiply substituted amines. Their results were published in the Journal of the American Chemical Society .
“Amines are present in many bioactive molecules, so being able to functionalize them using a benign catalyst and hydrogen is an attractive approach that will allow researchers to realize challenging molecular transformations,” Sensuke Ogoshi, one of the corresponding authors, comments “Until now, this has been a significant challenge; however, our method has demonstrated highly efficient synthesis of a wide variety of amines including amino acids.”
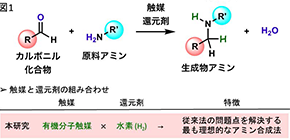
Their reductive alkylation method uses hydrogen directly, resulting in the generation of water as the only byproduct, which ensures that the method is atom-efficient and clean. In addition, their method can efficiently functionalize amines that have a wide range of substituents, including carboxyl, hydroxyl, additional amino, primary amide, and primary sulfonamide groups, which have proven to be challenging starting materials for previously reported procedures.
“The simple experimental procedure should broaden the scope of potential reaction substrates,” said Yoichi Hoshimoto, another corresponding author. “Our results can contribute to a rapid and efficient expansion of bioactive amine libraries.”
Greener synthetic methods should provide an opportunity for human society to more harmoniously coexist with the natural world. In this regard, the present environmentally benign process for direct functionalization of amino acids with hydrogen will pave the way for the future of chemical synthesis.
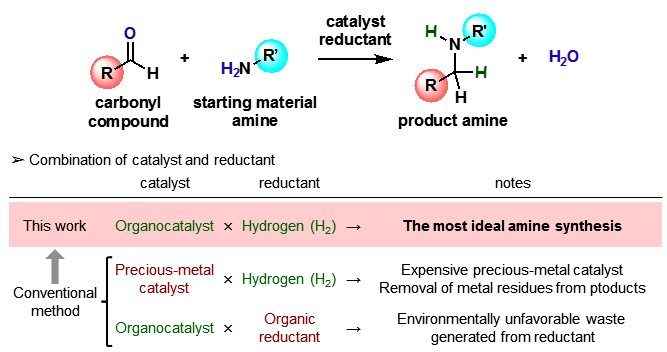
Figure 1. General scheme for catalytic reductive alkylation of amines
(credit: Osaka University)
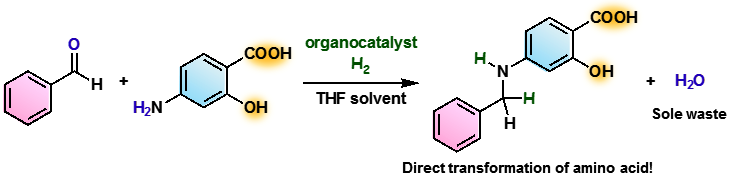
Figure 2. Example of the catalytic reductive alkylation in this work
(credit: Osaka University)
To learn more about this research, please view the full research report entitled "Main-Group-Catalyzed Reductive Alkylation of Multiply Substituted Amines with Aldehydes Using H 2 " a t this page of Journal of the American Chemical Society .
Related links
