The importance of the glutamine metabolism in colon cancer
A deft role for survival of cancer
Outline
The importance of glutamine was made clear as a colon cancer specific metabolism. It is known that glutamine metabolism is important for pancreatic cancer, but the importance of glutamine metabolism for colon cancer has been unclear. In this study, we showed the importance of glutamine metabolism.
Background
The importance of glutamine metabolism for colon cancer was revealed by Masamitsu Konno Ph.D, Masaaki Miyo M.D. Ph.D, Masaki Mori, M.D. Ph.D and Hideshi Ishii M.D. Ph.D. The aim of this study is to elucidate metabolic adaptation to nutritional stress and the role of the involved oncogenes in human colorectal cancer. The present study showed that the metabolism of colorectal cancer, distinct from that of pancreatic cancer, depended on genomic alterations, which previously have been uncharacterized, and was not restricted to KRAS mutation alone. Colorectal cancer can survive under the condition of glucose depletion while retaining TCA cycle activity. The cells’ survival relies on a delicate balance between energy and reactive oxygen species (ROS) production. Glutamate dehydrogenase 1 (GLUD1) and SLC25A13 have pivotal roles under glucose-deprived conditions and are associated with tumor aggressiveness and colorectal cancer prognosis.
Address to the society
GLUD1 and SLC25A13 have pivotal roles in nutritional stress and are associated with tumor aggressiveness and poorer prognosis of colorectal cancer. These proteins may serve as new targets in the treatment of refractory colorectal cancer.
Comments/messages from researcher(s)
We found that colorectal cancer cells survived under the condition of glucose depletion, and their resistance to such conditions depended on genomic alterations rather than on KRAS mutation alone. Metabolomic analysis demonstrated that those cells maintained TCA cycle activity and ATP production under such conditions. Furthermore, we identified pivotal roles of GLUD1 and SLC25A13 in nutritional stress. GLUD1 and SLC25A13 were associated with tumor aggressiveness and poorer prognosis of colorectal cancer. GLUD1 and SLC25A13 may serve as new targets in treating refractory colorectal cancer, which survives in malnutritional microenvironments.
Abstract
Tumor cells respond to their microenvironment, which can include hypoxia and malnutrition, and adapt their metabolism to survive and grow. Some oncogenes are associated with cancer metabolism via regulation of the related enzymes or transporters. However, the importance of metabolism and precise metabolic effects of oncogenes in colorectal cancer remain unclear. We found that colorectal cancer cells survived under the condition of glucose depletion, and their resistance to such conditions depended on genomic alterations rather than on KRAS mutation alone. Metabolomic analysis demonstrated that those cells maintained tricarboxylic acid cycle activity and ATP production under such conditions. Furthermore, we identified pivotal roles of GLUD1 and SLC25A13 in nutritional stress. GLUD1 and SLC25A13 were associated with tumor aggressiveness and poorer prognosis of colorectal cancer. In conclusion, GLUD1 and SLC25A13 may serve as new targets in treating refractory colorectal cancer which survive in malnutritional microenvironments.
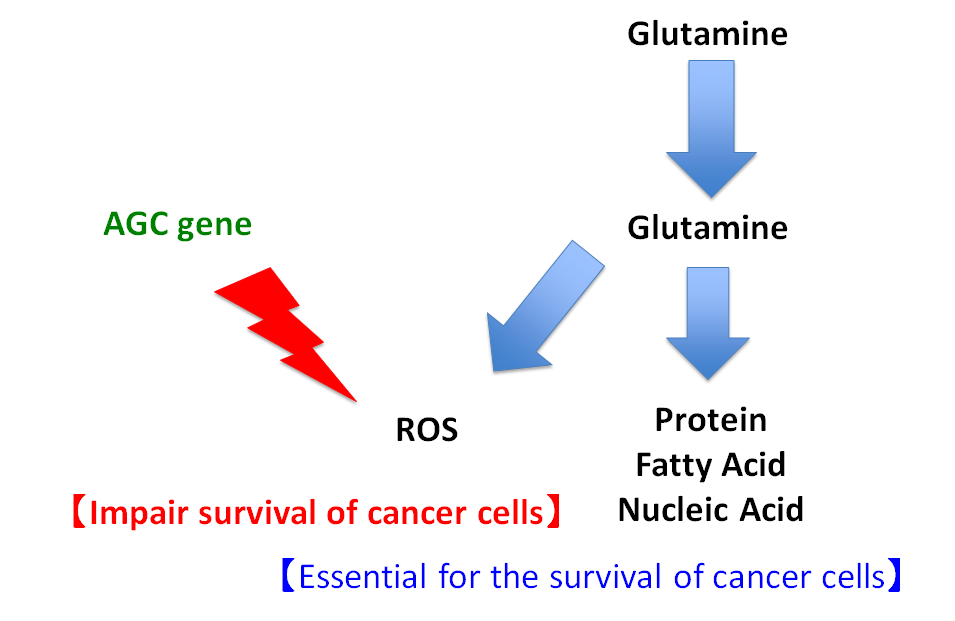
Figure 1. Colon cancer cells produce proteins, fatty acids, nucleic acids and reactive oxygen species (ROS). Proteins, fatty acids, and nucleic acids are essential factors for cell survival using glutamine metabolism. On the other hand, ROS is a factor for cellular disorder. Colon cancer cells up-regulate the expression AGC gene. Then AGC reduce ROS.
Figure 2
To learn more about this research, please view the full research report entitled “ Metabolic Adaptation to Nutritional Stress in Human Colorectal Cancer ” at this page of the Scientific Reports website.
Related link
