Crystal structure and control mechanism of human Ca2+ pump activity clarified
Will unveil how dysfunctional calcium homeostasis in cells causes diseases
A team of researchers from Tohoku University, Nara Institute of Science and Technology, Osaka University, Tohoku University, and Kyoto Sangyo University clarified the crystal structure of a calcium pump SERCA2b, which plays an important role in controlling muscle contraction and inducing apoptosis to maintain calcium homeostasis through the regulation of calcium ion concentration, a world first.
An endoplasmic reticulum (ER) is a subcellular organelle that plays a central role in the synthesis of membrane proteins and secreted proteins as well as calcium storage. SERCA2b membrane protein, an adenosine triphosphate (ATP)-driven calcium pump in an ER membrane, is present in all tissues to facilitate Ca 2+ uptake into the ER, thereby playing an important role in intracellular calcium homeostasis.
Because SERCA2b has a characteristic C terminal segment containing the 11th transmembrane helix (TM11), SERCA2b includes a characteristic 11th transmembrane helix (TM11) followed by a luminal tail. SERCA2b activity is regulated by the C terminal segment, but its detailed mechanism had not been clarified.
Using X-ray crystallography, this team determined the structure of SERCA2b: it consists of 3 cytosolic A, N, and P domains and 11 TMs (called “TM1~TM11” from the N terminal end). They found that TM11 was located at a completely different site from the previously predicted location, adjacent to TM10, identifying the binding sites and modes of adenylyl methylenediphosphonate (AMPPCP) and Mg 2+ ions in N domain, and two Ca 2+ ions in the TM helix region.
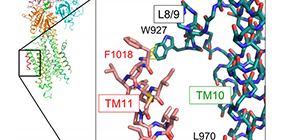
The crystal structure showed that TM11 interacted with a part of the L8/9 loop and TM10 in SERCA2b and amino acid mutation analyses clarified that these intermolecular interactions were important for regulating SERCA2b activity. In comparison with the steric structure of SERCA2a, they found that, in SERCA2b, TM11 restricted the movement of the TM helix region (TM1-10), regulating SERCA2b activity.
The team performed structural analysis of Ca 2+ -ATPase during the reaction cycle of SERCA2b, proposing regulatory mechanisms of SERCA2b activity through TM11-mediated intermolecular interactions. Knowledge obtained in this study will clarify cellular calcium-related biological phenomena and causes of and treatment strategies for various diseases caused by dysfunction of calcium homeostasis.
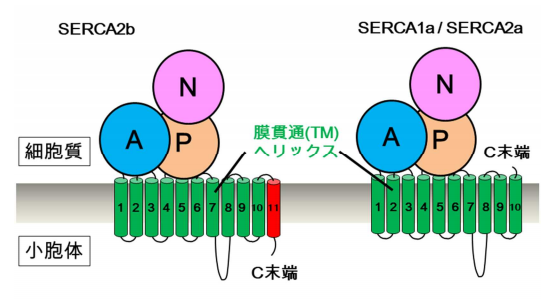
Figure 1
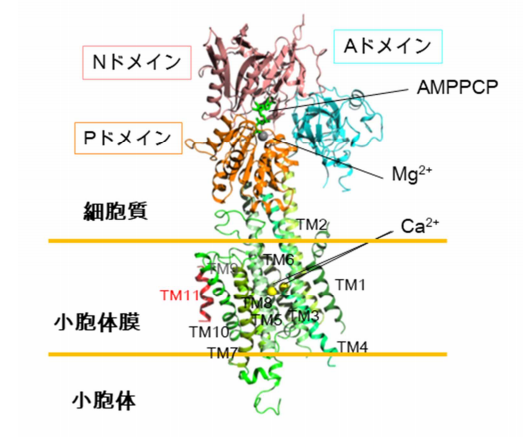
Figure 2
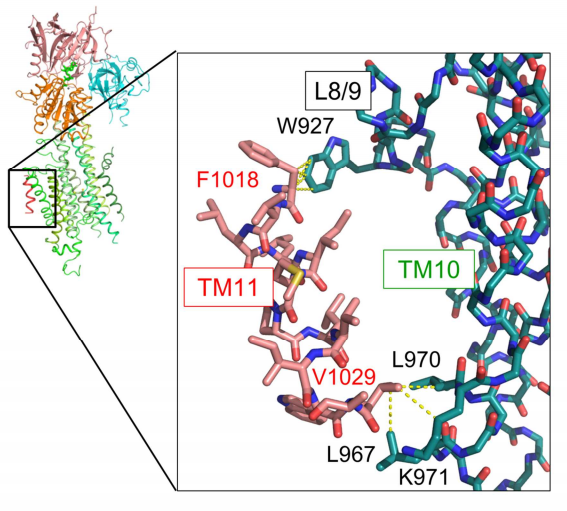
Figure 3

Figure 4
The article, “Structural basis of sarco/endoplasmic reticulum Ca 2+ -ATPase 2b regulation via transmembrane helix interplay” was published in Cell Reports at DOI: https://doi.org/10.1016/j.celrep.2019.03.106 .
Related links
