Mass-production of proteins necessary for maintenance and proliferation of stem cells, the key to regenerative medicine, established
A new method for safe mass-production with no impurities
A group of researchers led by TAKAGI Jun’ichi (Professor, Institute for Protein Research) found that Wnt forms a 1:1 complex with Afamin (AFM), also known as α-albumin . Wnt is a protein necessary for the generation and tissue formation of various multicellular organisms including humans, and AFM is a protein in the blood. Using this phenomenon, this group developed a new method for purifying and storing Wnts.
Wnt proteins act on cells and control their fate, and are involved in various diseases, such as cancer and osteoporosis. Unlike ordinary secreted proteins, they are not soluble in water, so it was difficult to prepare them in an active state for medical or research purposes.
By making AFM-Wnt complexes, Professor Takagi and MIHARA Emiko (Specially Appointed Researcher) succeeded in preparing Wnt proteins without adding additives. It was thought that the purification of Wnt proteins couldn’t be achieved without surface acting agents.
In cooperation with research groups from Osaka University’s Graduate School of Medicine and Keio University, this group verified that AFM-Wnt complexes have bioactivity against cells.
Wnt proteins are a factor necessary for increasing a small amount of stem cells in vivo and iPS cells in vitro. This group’s achievement has enabled the preparation of Wnts in large amounts and without impurities, which surely will accelerate regenerative medicine research.
Wnt plays important role during development and in various diseases. Because Wnts are lipidated and highly hydrophobic, they can only be purified in the presence of detergents, limiting their use in various in vitro and in vivo assays. We purified N-terminally tagged recombinant Wnt3a secreted from cells and accidentally discovered that Wnt3a co-purified with a glycoprotein afamin derived from the bovine serum included in the media. Wnt3a forms a 1:1 complex with afamin, which remains soluble in aqueous buffer after isolation, and can induce signaling in various cellular systems including the intestical stem cell growth assay. By co-expressing with afamin, biologically active afamin-Wnt complex can be easily obtained in large quantity. As afamin can also solubilize Wnt5a, Wnt3, and many more Wnt subtypes, afamin complexation will open a way to put various Wnt ligands and their signaling mechanisms under a thorough biochemical scrutiny that had been difficult for years.

Wnt3a in complex with AFM is biologically active.
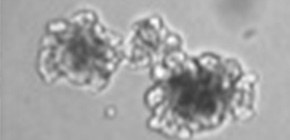
( A ) Comparison between the purified Wnt3a from a commercial source and the AFM-Wnt3a complex. Increasing concentrations of Wnt3a proteins are added to TCF reporter cells and the Wnt signaling activities are evaluated as in Figure 1B . The data are mean ± SD of three independent experiments, in which quadruplicate determinations were made. See Figure 5—source data 1 . ( B ) AFM-Wnt3a complex supports self-renewal of human intestinal stem cells. The ability of single-cell dissociated human intestinal organoids to expand was evaluated by culturing in the absence (control) or presence of various forms of Wnt3a preparations. Photographs are taken every 7 days at a low (x2, entire view of one 48-well, upper panels) and the high (x 10, lower panels) magnifications. Bar: 200 µm.
To learn more about this research, please view the full research report entitled " Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin " at this page of the eLIFE website.
Related link
- Institute for Protein Research, Osaka University (link in Japanese)
