Genetic control of immune cell proliferation
Members at IFReC, Osaka University provide new insights on why the tumor suppression gene Foxo1 can actually be the cause of some cancers
Germinal centers are transient structures in the lymph nodes where antibody-producing B cells proliferate and differentiate at extraordinary rates. Germinal centers can be visually divided into a dark zone and light zone. For the proliferation and differentiation to occur, B cells must cycle between the two zones. Investigators at the Immunology Frontier Research Center (IFReC), Osaka University have discovered how specific genes regulate this cycling. The findings, which can be read in the Journal of Experimental Medicine , provide new insights on how certain types of lymphomas form.
To understand how B cells cycle in the germinal center, Prof. Tomohiro Kurosaki, who led the project, set his eyes on Foxo1.
“Foxo1 is a tumor suppressor gene, but it promotes B cell proliferation in the germinal center,” he said.
Normally, the suppression of Foxo1 expression enables cells to proliferate. Even B cells outside the germinal center will proliferate when Foxo1 is suppressed. However, some lymphomas are associated with activated Foxo1, and Kurosaki and his team observed that inside germinal centers, B cells with Foxo1 suppressed will actually lower in numbers.
Transferring the mutant B cells into mice, the researchers further showed that Foxo1 has a vital role in the cycling of B cells between light and dark zones.
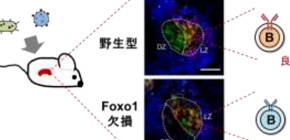
“We found B cells remained in the light zone,” said Kurosaki of the Foxo1-deficient B cells.
The dark zone is where B cells undergo proliferation. When in the light zone, B cells are selected by follicular helper T (T FH ) cells for migration to the dark zone. Kurosaki found that without Foxo1, B cells are not selected at all.
"Knocking out Foxo1 reduced the expression of BCR," he said.
BCR, or B cell receptors, describe the unit on the B cell that binds to the invasion and from which B cells are selected to proceed with the cycling. Moreover, even if the Foxo1-deficient B cells could be manipulated to be selected by the T FH cells, they still did not proliferate, suggesting another factor besides Foxo1 is also imperative to cycling.
Kurosaki’s team noticed that the knockout of Foxo1 also led to a reduction in BATF expression in B cells. Reviving the BATF levels recovered the proliferation of Foxo1-deficient B cells in the germinal center. Thus, the proliferation deficiency could be the result of poor crosstalk between the two genes.
The findings provide important new knowledge on antibody production by the body and also the development of certain cancers.
“Even though Foxo1 is a tumor suppressor gene, paradoxically its activity is associated with lymphomas,” said Kurosaki. This study provides new candidate molecular targets for the treatment of such lymphomas.
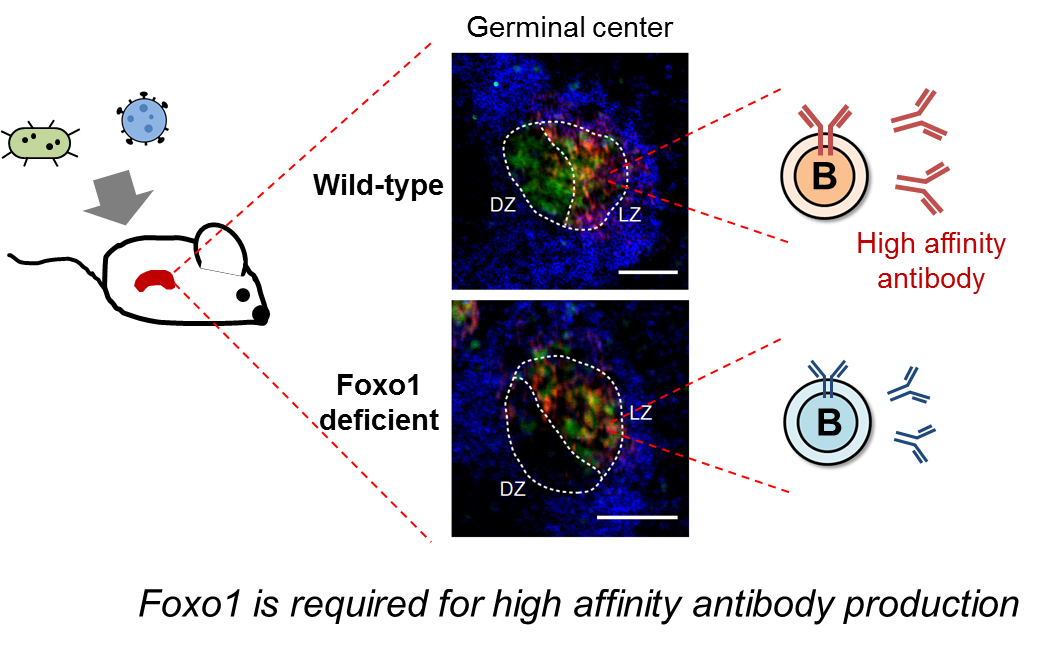
Figure 1: Without Foxo1, germinal center structure is disorganized and mice could not produce high affinity antibodies against foreign antigens.

Figure2: Dark zone is lost and the germinal center is collapsed upon Foxo1 ablation. Foxo1 has also important roles in the light zone B cells, including antigen presentation to TFH cells and expression of dark zone program genes.
To learn more about this research, please view the full research report entitled " The transcription factor Foxo1 controls germinal center B cell proliferation in response to T cell help " at this page of The Journal of Experimental Medicine.
Related link
