Local changes of intracellular calcium ion concentration play an important role in developing the brain
Researchers at the National Institute for Basic Biology, together with those from Kyoto University, The Institute of Scientific and Industrial Research, Osaka University, and University of Alberta (Canada), clarified that transient and local changes of intracellular calcium ion (Ca 2+ ) concentration caused cell shape change, playing an important role in developing the brain.
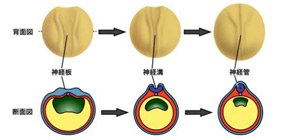
The brain and spinal marrow are formed in an early development stage from a fertilized egg. The shape of cells changes from a columnar to a bottle shape or a wedge shape. This cellular morphology is called apical constriction. If this process is disrupted, neural tubes cannot close correctly, which leads to a severe congenital malformation called neural tube defects.
Previously, this group had studied the formation of neural tubes by using the African clawed frog, Xenopus laevis, whose process of forming the brain is similar to that of humans, and discovered that intracellular calcium ion (Ca 2+ ) concentration changes in the chorda, and other tissues were involved in controlling formation and movement of cells.
This time, focusing on Ca 2+ -concentration changes in neural tube formation, this group performed experiments by using pharmacological treatments to inhibit Ca 2+ -concentration changes and found that the treatments hampered Xenopus neural tube formation. Next, this group observed the groups of cells that form neural tubes by using fluorescent protein GECO (Genetically-Encoded Ca 2+ indicators for Optical imaging), whose brightness changes dependently to intracellular Ca 2+ , and discovered transient drastic increases in intracellular Ca 2+ in some cell groups. This group also found that following intracellular Ca 2+ -concentration changes, the distribution of cycroskeletal actin filaments (F-actin), which is involved in a cell shape change, drastically changed, inducing apical constriction.
This group changed Ca 2+ concentration by using technology for inducing an increase in intracellular Ca 2+ -concentration with light, which caused apical constriction. From this, it was suggested that intracellular Ca 2+ caused apical constriction through changes of F-actin dynamics, accelerating the closing movement of the neural plate. Furthermore, this group demonstrated that intracellular Ca 2+ concentration was controlled by stimulation by extracellular adenosine triphosphate (eATP), clarifying a part of control mechanism behind a cell shape change in neural tube formation.
This group’s achievements clarified a part of mechanism behind Xenopus neural tube formation. Apical constriction mechanism and the process of neural tube formation are conserved in vertebrates, so this group’s achievements will lead to general understanding of brain development in vertebrates, including humans.
Abstract
Early in the development of the central nervous system, progenitor cells undergo a shape change, called apical constriction, that triggers the neural plate to form a tubular structure. How apical constriction in the neural plate is controlled and how it contributes to tissue morphogenesis are not fully understood. In this study, we show that intracellular calcium ions (Ca 2+ ) are required for Xenopus neural tube formation and that there are two types of Ca 2+ -concentration changes, a single-cell and a multicellular wave-like fluctuation, in the developing neural plate. Quantitative imaging analyses revealed that transient increases in Ca 2+ concentration induced cortical F-actin remodeling, apical constriction and accelerations of the closing movement of the neural plate. We also show that extracellular ATP and N-cadherin (cdh2) participate in the Ca 2+ -induced apical constriction. Furthermore, our mathematical model suggests that the effect of Ca 2+ fluctuations on tissue morphogenesis is independent of fluctuation frequency and that fluctuations affecting individual cells are more efficient than those at the multicellular level. We propose that distinct Ca 2+ signaling patterns differentially modulate apical constriction for efficient epithelial folding and that this mechanism has a broad range of physiological outcomes.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
To learn more about this research, please view the full research report entitled " Distinct intracellular Ca 2+ dynamics regulate apical constriction and differentially contribute to neural tube closure " at this page of the Development website.
Related links
