Precise Inactivation of Neural Messenger Receptor Wipes Out Fear Memory in Mice
Research combines antibody precision with toxic oxygen burst to inactivate neural protein and temporarily abolish fear memory in mice
The delivery of chemical messenger (neurotransmitter) receptors to the junctions between nerve cells (synapses) is crucial to cognitive processes such as memory. One way of understanding the function of these receptors is to inactivate them and observe the outcome. However, this is only informative if the inactivation is precise with respect to space and time. Many techniques used to block receptor functions affect both cell surface and internal forms of the proteins, yet neurotransmitter receptors typically work at the cell surface. Work at Japanese institutions, including Yokohama City University, Osaka University and the University of Tokyo, modified a light-induced means of producing a burst of destructive oxygen (CALI: chromophore-assisted light inactivation) by incorporating an antibody to achieve specificity in protein inactivation. The study was reported in Nature Biotechnology .
The technique known as CALI has previously been applied to investigate protein functions. It uses light irradiation to generate a temporary toxic form of oxygen that causes an area of damage shorter than a typical protein–protein interaction distance. In the present work, researchers made an antibody against the outer part of the neurotransmitter receptor GluA1 that they labeled with a light-sensitive molecule (a photosensitizer). The antibody provided the necessary specificity to inactivate GluA1 receptor synapse responses both in cultured cells and in vivo in mice.
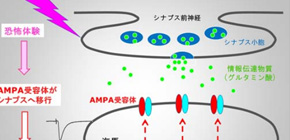
The team injected the labeled antibody into the hippocampus, a region of the brain involved in memory and navigation, of mice. They then assessed its effect on memory formation by using a fear-learning task in which mice move between light and dark boxes, receiving an electric foot shock in the dark boxes only so they learn to favor the light boxes. This task was shown by the team to require the delivery of GluA1 to synapses in the rat hippocampus in an earlier study.
“In response to illumination of the mouse hippocampus with green light, we found that mice returned to the dark boxes more quickly than control animals,” study first author Kiwamu Takemoto says. “This showed that the fear memory had been erased by the inactivation of synaptic GluA1.”
The specificity of the process for the GluA1 type of receptor was shown by varying the time at which CALI was performed after the mice first experienced the fear-learning task. Administering CALI up to 2 hours after the first task resulted in electrical activity representative of the delivery of GluA1 receptors to synapses. However, this activity was undetectable 24 hours after the first task. The researchers interpret this as evidence for the replacement of GluA1 receptors by receptors containing the related protein GluA2, which is consistent with the fact that mice treated with CALI at the 24-hour time point do not lose their fear memory.
Abstract
The synaptic delivery of neurotransmitter receptors, such as GluA1 AMPA ( α -amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors, mediates important processes in cognitive function, including memory acquisition and retention. Understanding the roles of these receptors has been hampered by the lack of a method to inactivate them in vivo with high spatiotemporal precision. We developed a technique to inactivate synaptic GluA1 AMPA receptors in vivo using chromophore-assisted light inactivation (CALI). We raised a monoclonal antibody specific for the extracellular domain of GluA1 that induced effective CALI when conjugated with a photosensitizer (eosin). Mice that had been injected in the CA1 hippocampal region with the antibody conjugate underwent a fear memory task. Exposing the hippocampus to green light using an implanted cannula erased acquired fear memory in the animals by inactivation of synaptic GluA1. Our optical technique for inactivating synaptic proteins will enable elucidation of their physiological roles in cognition.
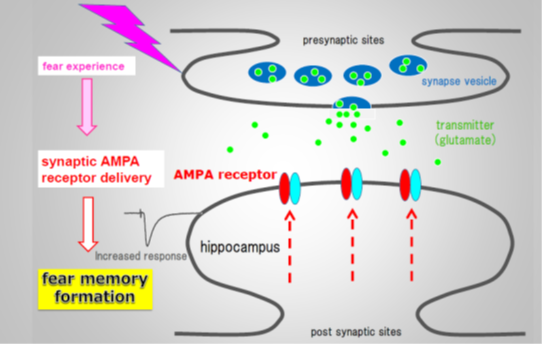
Figure 1. Fear memory formation drives AMPA receptors into synapses

Figure 2. Optical inactivation of AMPA receptors erases fear memory.
To learn more about this research, please view the full research report entitled “Optical inactivation of synaptic AMPA receptors erases fear memory” at this page of the Nature Biotechnology website.
Related link
