Superior pathological diagnosis using transparent tissues
RIKEN Quantitative Biology Center and Osaka University researchers show CUBIC, a tissue clearing and 3D imaging technique, makes human organs transparent to improve pathology diagnosis
To some, the idea of invisibility leads to mischief, seeing things without being seen. To the pathologist, the idea of invisibility leads to simplicity, seeing the disease in its fullest. In a new study published in Scientific Reports , Japanese researchers report, CUBIC, a technique including a tissue processing that makes human organs transparent, provides better assessment of lesions for pathological diagnosis.
“Traditionally, pathological diagnosis is made by taking 2D sections of a specimen resected from patients. It is effective, but we cannot exclude the possibility that important findings away from the cut surface are overlooked,” explains Osaka University Professor Eiichi Morii, who co-corresponding-authored the study.
Contemporary methods are based on staining techniques from the 19 th Century. Patient specimens are cut into thin sections that are stained and analyzed individually under a microscope. However, this method has limitations in its relatively narrow range of observation area and in its two-dimensionality. CUBIC (Clear, Unobstructed Brain/Body Imaging Cocktails and Computational Analysis) was first reported by RIKEN Group Director Hiroki R. Ueda, who is the corresponding author of this study, and his colleagues three years ago. CUBIC has been used to observe whole organs mainly from experimental animals. In the new study, Ueda and his colleagues demonstrated that CUBIC can be used to observe organs from humans and that it surpasses current methods for pathological diagnosis/study.
The study shows CUBIC applicability to the 3D imaging of patient lung and lymph node tissues, clearly delineating normal and abnormal regions (Figure 1, Figure 2). After routine observation, many patient samples are stored at hospitals as paraffin-embedded tissue blocks. Additionally, the study shows that the combination of appropriate deparaffinization and CUBIC enabled 3D imaging of these older specimens.
“These results mean that we can use not only newly fixed samples but also paraffin-embedded tissues stored in the pathological archives of hospitals,” said Osaka University Assistant Professor Satoshi Nojima, who first-authored the study.
The scientists also examined the practical diagnostic potential of CUBIC. They showed that CUBIC was much more capable of detecting metastatic carcinomas in lymph node specimens compared to standard pathology techniques (Figure 3).
“This is outstanding result to demonstrate the usefulness of CUBIC on practical clinical examination,” Nojima said.
These findings show the potential of CUBIC for retrospective and prospective clinicopathological diagnosis.
“Our wish is to improve CUBIC so that it leads to the establishment of a novel field of medical science based on 3D histopathology.” Ueda said.
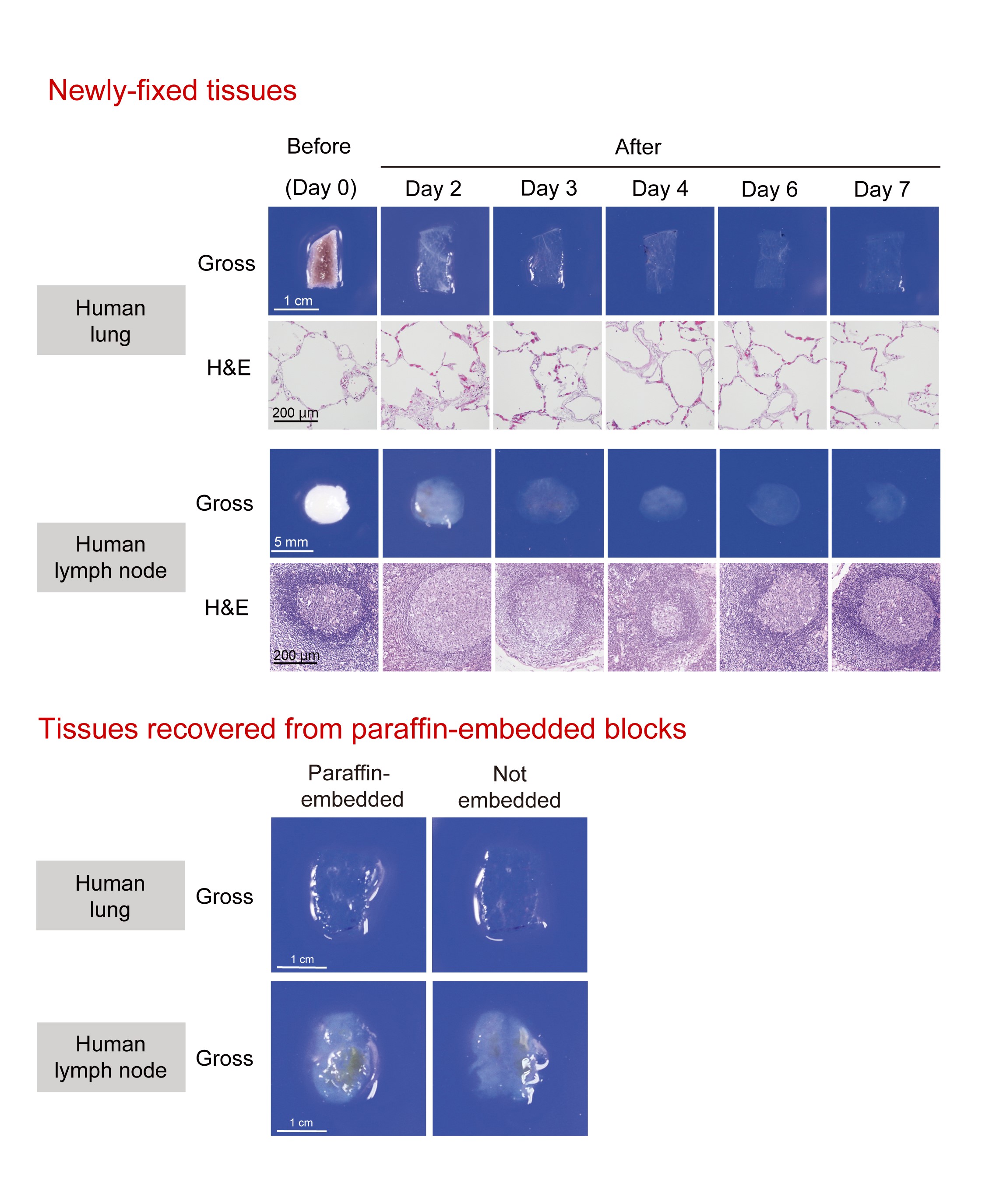
Fig.1. Tissue clearing of various human tissues with CUBIC.
(Upper) Gross and microscopic images of human lung and lymph nodes cleared by CUBIC. After gross image acquisition at the indicated time points, lung and lymph node tissues were washed with PBS, followed by paraffin embedding, sectioning, and H&E staining. These images show that lung and lymph node tissues were significantly cleared and that CUBIC clearing caused only negligible, if any, degeneration of the tissues. (Lower) Tissue blocks from formaldehyde-fixed normal human lung and lymph node were cut in half. One half was kept in PBS (“Not embedded”) whereas another half was first embedded into a paraffin block, was recovered by deparaffinization (“Paraffin-embedded”), and then was subjected to tissue clearing by CUBIC. The gross appearance of the resulting samples was not distinguishable and had an equivalent transparency. (©2017 Nojima S. et al. Scientific Reports, 7:9269, 1-14. DOI: 10.1038/s41598-017-09117-0)

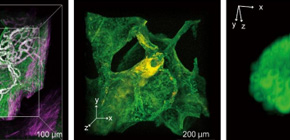
Fig. 2. 3D imaging of pathological specimens with CUBIC.
(Left) The reconstructed 3D image of a normal human lung tissue stained with green-fluorescent nucleic acid stain SYTO 16 and Alexa Fluor 647-conjugated anti-α-SMA (smooth muscle actin) antibody, showing the network of vasculature in the pulmonary alveolar interstitium. The image was obtained by confocal fluorescence microscopy. (Middle) The reconstructed 3D image of a lung tissue derived from an amyloidosis patient. The tissue was stained with SYTO 16 and Congo Red, showing the amyloid deposition in artery wall in lung. The image was obtained by confocal fluorescence microscopy. (Right) The reconstructed 3D image of SYTO 16-stained half-cut human mesenteric lymph node. The image was obtained by light-sheet fluorescence microscopy. (©2017 Nojima S. et al. Scientific Reports, 7:9269, 1-14. DOI: 10.1038/s41598-017-09117-0)
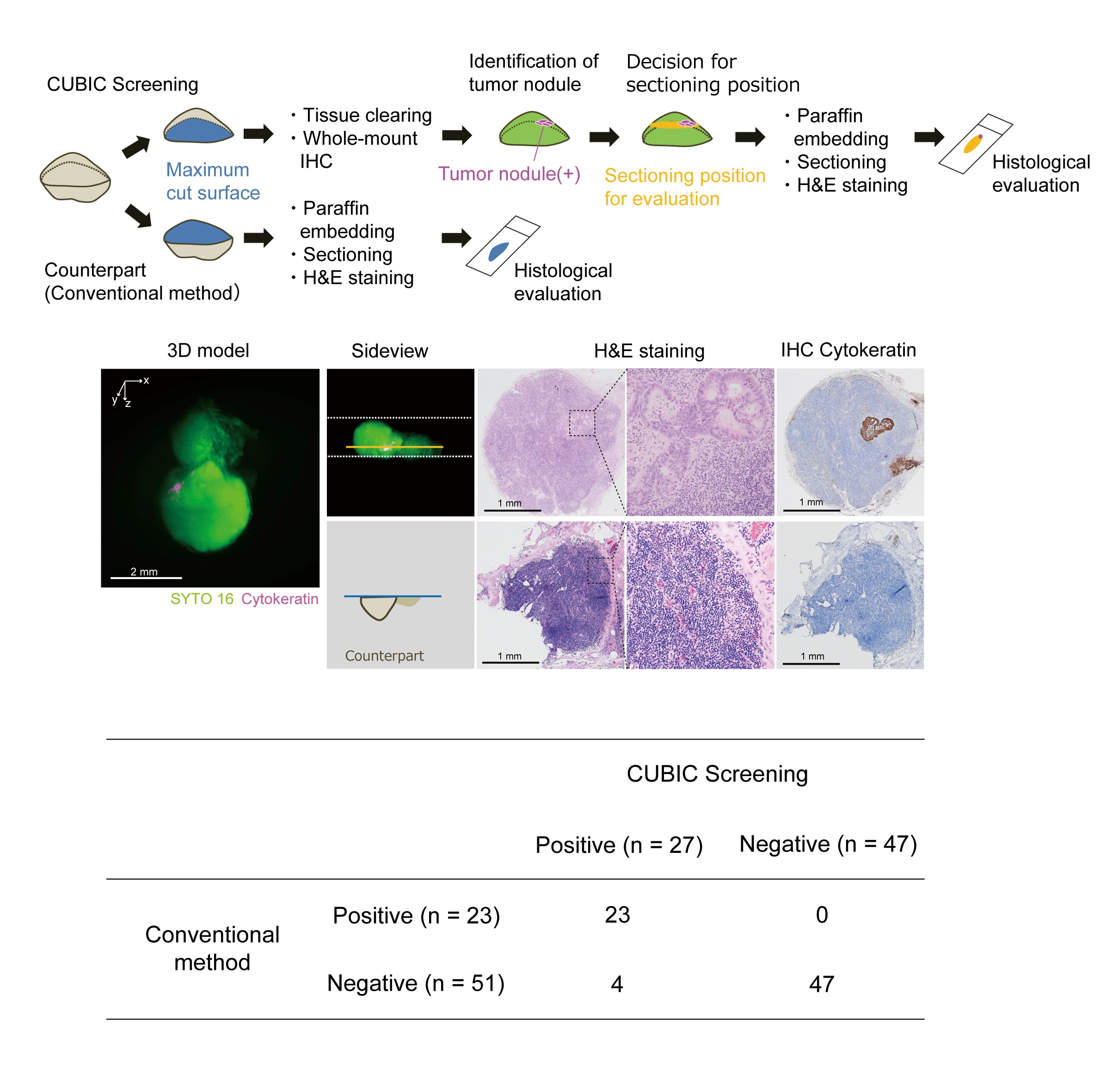
Fig.3. Screening of metastatic carcinoma nodules in lymph nodes with CUBIC.
(Upper) Schematic diagram of the metastasis screening protocol. (Middle) The representative images of reconstructed 3D model, H&E staining, or Cytokeratin immunohistochemical (IHC) staining of the lymph node contained small carcinoma nodules which were only identified by CUBIC screening but were not detected by routine diagnosis method. (Lower) The results of screening are summarized in the table. Four lymph nodes out of 74 were newly diagnosed as positive for metastasis only by the CUBIC-based screening, making the sensitivity of the conventional diagnosis method 85.2% (23/27) with four false negative, i.e. 14.8% (4/27) improvement in sensitivity with CUBIC. (©2017 Nojima S. et al. Scientific Reports, 7:9269, 1-14. DOI: 10.1038/s41598-017-09117-0)
To learn more about this research, please view the full research report entitled " CUBIC pathology: three-dimensional imaging for pathological diagnosis " at this page of Scientific Reports .
Related links
