Discovery of Molecular Marker Specific to Early Embryonic Heart Development
Will lead to development of regeneration and cell replacement therapy for heart failure
Regenerative medicine aiming for effective treatment by transplanting cardiomyocytes made from human pluripotent stem cells such as ES cells and iPS cells is attracting attention. Additionally, facilitating personalized medicine through regenerative medicine, that is, clarifying a pathological mechanism by creating disease models by using cardiomyocytes created from patients’ iPS cells, confirming effects of drugs on cultured dishes, and adjusting the combination of more effective drugs according to individual patients, is also sought after. However, since markers used for sorting out cardiac progenitors (CPs) were not specific, it was difficult to isolate cells to be differentiated into cardiomyocytes from cells that made up the heart.
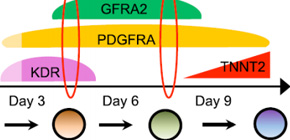
A group of researchers led by Specially Appointed Assistant Professor ISHIDA Hidekazu, Specially Appointed Associate Professor YASHIRO Kenta, and Professor OZONO Keiichi at the Graduate School of Medicine, Osaka University, discovered a surface marker, GFRA2, as a marker of cardiomyocyte precursors (cell surface antigens).
By using GFRA2 as a marker, it has become possible to isolate cardiomyocyte precursors, cells in the previous stage of cardiomyocytes induced from ES cells and iPS cells, with great accuracy. Furthermore, this group clarified in animal experiments that GFRA2 might cause severe myocardial disease called myocardial noncompaction when it was abnormal in function.
It is thought that it will become possible to isolate and examine CPs that create components of the heart, contributing to research on the process of heart development. It is expected that distinctive use of CPs with multilineage potential and CPs to be differentiated into cardiomyocytes only as markers will accelerate the development of drug test tools using ES and iPS cells as well as their applications in regenerative medicine.
Abstract
A surface marker that distinctly identifies cardiac progenitors (CPs) is essential for the robust isolation of these cells, circumventing the necessity of genetic modification. Here, we demonstrate that a Glycosylphosphatidylinositol-anchor containing neurotrophic factor receptor, Glial cell line-derived neurotrophic factor receptor alpha 2 (Gfra2), specifically marks CPs. GFRA2 expression facilitates the isolation of CPs by fluorescence activated cell sorting from differentiating mouse and human pluripotent stem cells. Gfra2 mutants reveal an important role for GFRA2 in cardiomyocyte differentiation and development both in vitro and in vivo. Mechanistically, the cardiac GFRA2 signaling pathway is distinct from the canonical pathway dependent on the RET tyrosine kinase and its established ligands. Collectively, our findings establish a platform for investigating the biology of CPs as a foundation for future development of CP transplantation for treating heart failure.
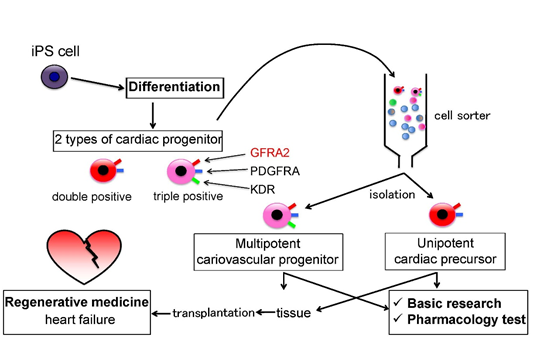
Medical usage of specific surface antigen GFRA2
Using GFRA2 as a cell surface marker, cardiac precursor cells can be sorted for basic research of cardiomyocyte, pharmacoloty test, or regenerative medicine of heart failure.
To learn more about this research, please view the full research report entitled “ GFRA2 identifies cardiac progenitors and mediates cardiomyocyte differentiation in a RET-independent signaling pathway ” at this page of the Cell Reports website.
Related link
