Multidrug resistance in bacteria: What causes it?
Structure of multidrug efflux pumps clarified
A group of researchers from the Institute for Protein Research of Osaka University examined MexAB-OprM, a multidrug efflux protein expressed in the Pseudomonas aeruginosa ( P. aeruginosa ) by cryo-electron microscopy (cryo-EM) single particle analysis, revealing its complex formation and drug efflux mechanism.
Nosocomial infections in patients with immunodeficiency diseases due to multidrug-resistant P. aeruginosa causes serious symptoms, constituting a social problem. Multidrug-resistance of P. aeruginosa is caused by a multidrug efflux protein, which is a multidrug efflux pump consisting of a resistance-nodulation-cell division (RND) transporter that penetrates the inner membrane and plays a major role in drug efflux.
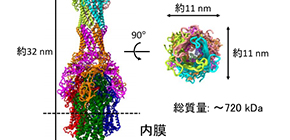
An RND efflux pump is composed of a membrane fusion protein (MexA), an inner membrane transporter (MexB), and an outer membrane channel (OprM). The structures of the components of MexAB–OprM have been solved individually; however, how these three types of proteins form a complex to release unwanted toxic substances that entered the bacterium had not been known.
The team mixed purified MexA, MexB, and OprM, reconstructing MexAB–OprM, a complex that penetrates both membranes, analyzing the structure of the complex by cryo-EM single particle analysis. They found the amino-acid residues essential for complex formation and the efflux route for drugs from the determined structure, confirming that drug resistance decreased in functional analysis using mutations.
This team’s achievements will make it easier to design chemical compounds that suppress bacterial drug efflux, which will lead to the development of antibiotics for multidrug-resistant organisms based on this newly found mechanism. Especially in Pseudomonas aeruginosa , MexAB–OprM plays a central role in multidrug resistance by ejecting various drug compounds. If MexAB–OprM inhibitors are produced, antibiotics that were previously released will activate in bacteria, which will become a silver bullet against multidrug-resistant P. aeruginosa .

Figure 1
The Article, “Structures of the wild-type MexAB-OprM tripartite pump reveal its complex formation and drugefflux mechanism” was published in Nature Communications at DOI: https://doi.org/ 10.1038/s41467-019-09463-9 .
Related links
