Method of the consecutive formation of bonds of 4 components
Can control organic catalyst reactions by using catalysts differently
A group of researchers led by Professor KAMBE Nobuaki and Assistant Professor IWASAKI Takanori at the Graduate School of Engineering, Osaka University developed a method of the consecutive formation of bonds of two butadiene, alkyl groups, and benzene rings by using cheap nickel catalysts.
Multicomponent reactions are methods which are superior in economy and efficiency to methods of bonding molecules by repeating reactions, but it was necessary to control the number of molecules to be bonded and locations of the bonds, so their applications were limited.
This group developed a synthesis method by constructing carbon frameworks of 8 carbons through the formation of bonds of two butadiene molecules by using cheap nickel catalysts and introducing alkyl groups and benzene rings to internal carbon bonds. Using this technique, it has become possible to synthesize high-value terminal olefin by using cheap butadiene. Using the same butadiene used in a wide range of fields as materials for synthetic rubber, such as tires and rubber hoses, and as materials for important industrial chemical compounds, such as butanediol and chloroprene, this group succeeded in making a variety of organic molecules by using different catalysts. This group’s achievement will lead to the development of methods for synthesizing various organic materials.
Furthermore, it is possible to synthesize butadiene from ethanol. This group’s achievement demonstrates the possibility of changing high-value chemical compounds such as bioethanol, which has been gathering attention in recent years, into different chemical compounds by using catalysts differently.
Abstract
In the presence of a nickel catalyst, 1,3-butadiene undergoes selective dimerization and alkylarylation with alkyl fluorides and aryl Grignard reagents to give 1,6-octadienes with alkyl and aryl groups at the 3- and 8-positions, respectively, by the consecutive formation of three carbon-carbon bonds. The formation of an anionic nickel complex plays an important role in forming C-C bonds with alkyl fluorides.
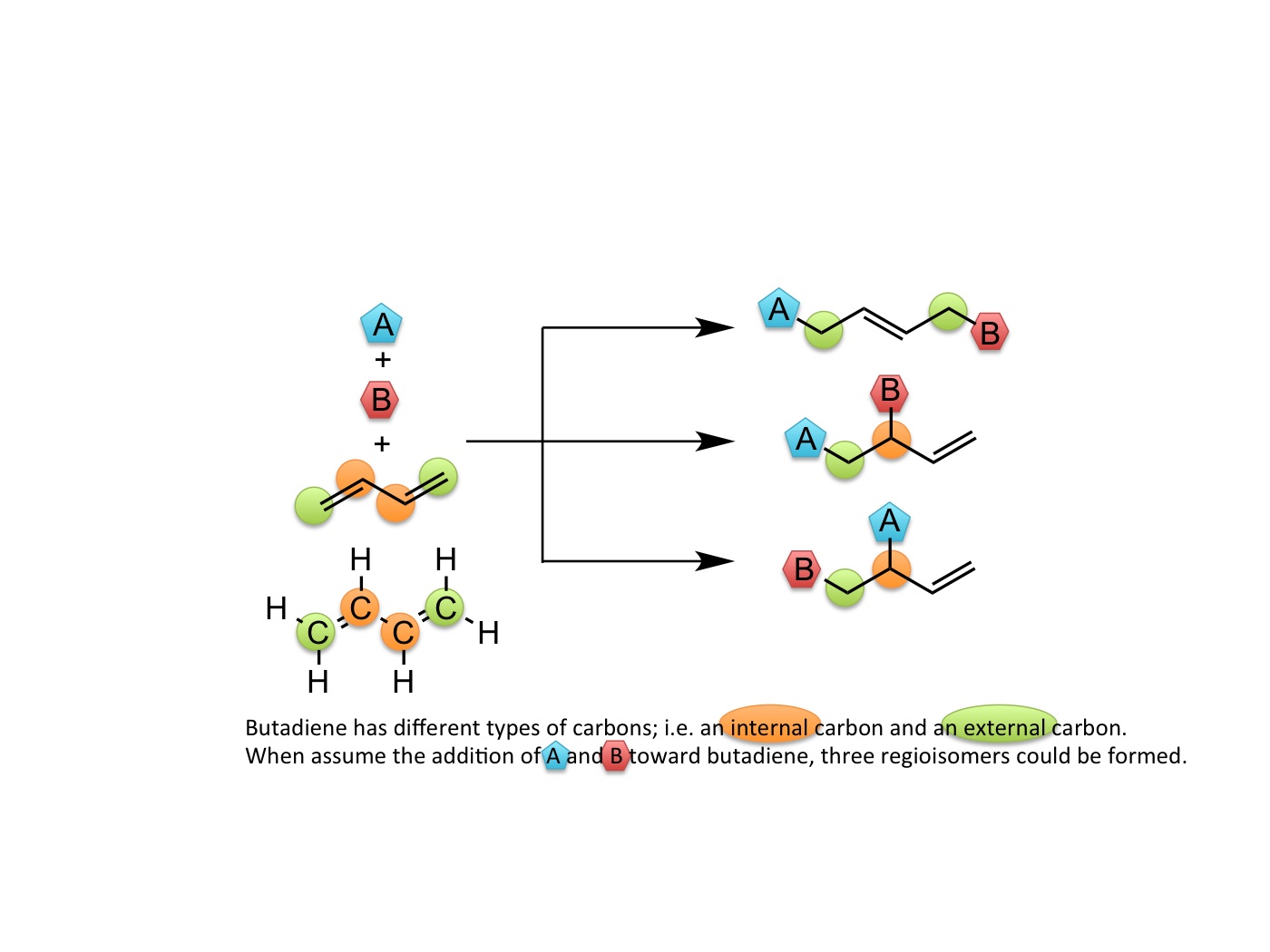
Figure 1. Possible products in the multicomponent reaction of butadiene
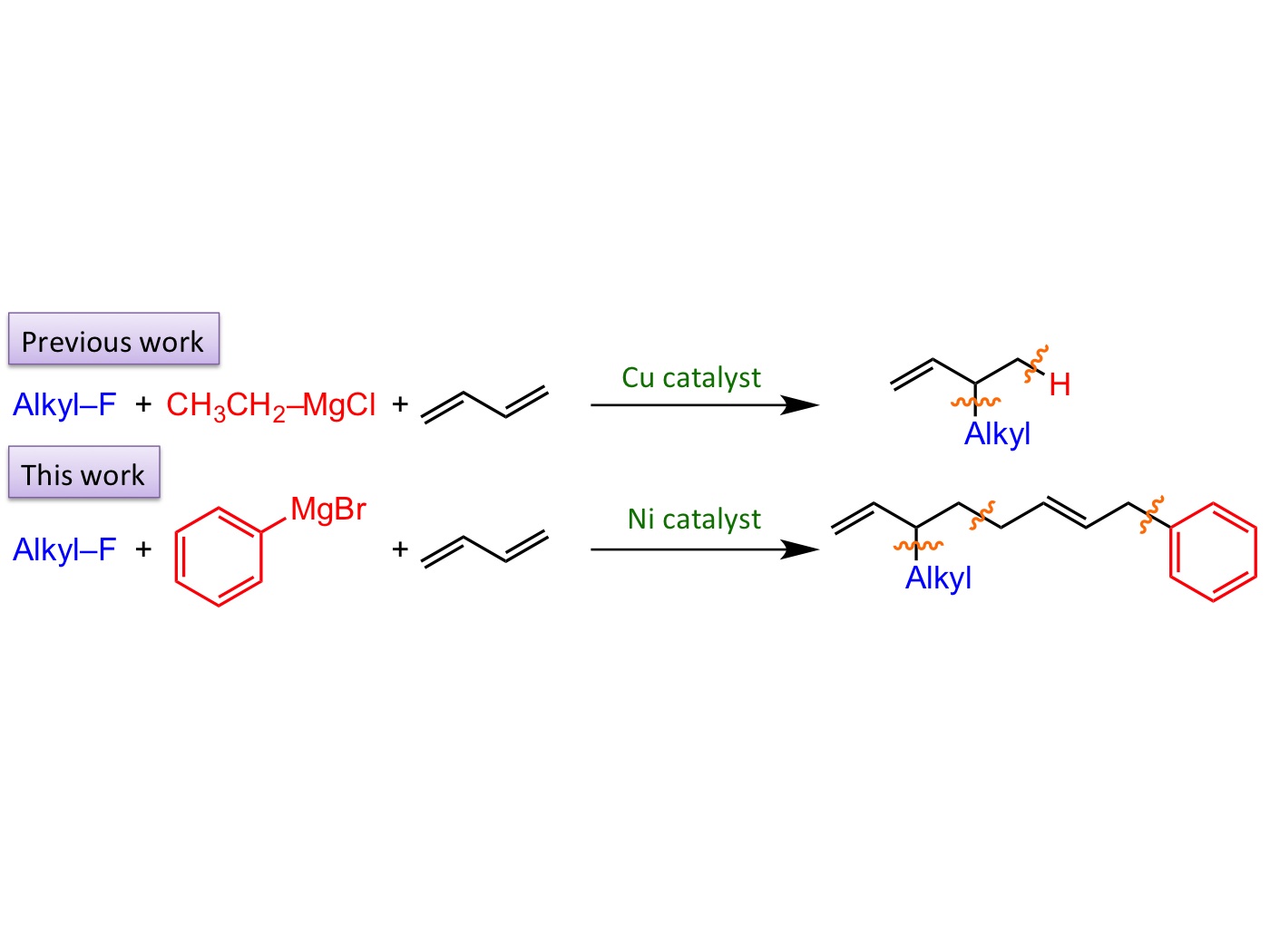
Figure 2. Comparison of the present Ni catalysis and previous Cu catalysis
in the alkylation of butadiene: wavy lines indicate newly formed C-C bonds
To learn more about this research, please view the full research report entitled " Nickel-Catalyzed Dimerization and Alkylarylation of 1,3-Dienes with Alkyl Fluorides and Aryl Grignard Reagents " at this page of the Angewandte Chemie International Edition website.
Related link
