Osaka chemists build new chemical structures on unreactive bonds
Osaka University research team develops new synthetic tool for building complex carbon frameworks by reacting strong carbon-fluorine bonds
Making complicated organic molecules is like solving a Rubik’s cube. Organic chemists need to design sequences of reactions to carefully build up parts of a molecule, while maintaining the structure at other sites. Although chemists have developed many ingenious ways of performing chemical transformations, some chemical reactions remain out of reach.
At Osaka University, a team of organic chemists has now developed and enhanced a chemical reaction that allows controlled transformations of one of the toughest chemical bonds.
“We previously developed a cobalt catalyzed Grignard reaction for making hindered quaternary carbon centers. But that reaction also showed potential for modifying carbon-fluorine bonds. We tried many different additives and eventually found one that let us selectively build the same quaternary carbon-carbon bonds at carbon-fluorine sites,” says first author Takanori Iwasaki.
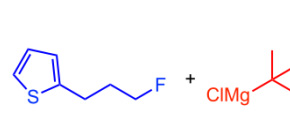
The Grignard reaction is a classic reaction in organic chemistry, useful for building the carbon skeleton of molecules by transforming carbon-halogen bonds into carbon-carbon bonds. Fluorine is also considered to be a halogen but the carbon-fluorine bond is among the strongest known and is usually unreactive to Grignard chemistry. Performing any kind of chemical reaction at carbon-fluorine bonds is difficult without affecting the rest of the molecule.
The Osaka team enhanced their catalytic system for performing difficult Grignard chemistry at very crowded, so-called quaternary carbon atoms. By adding a carefully selected additive to this catalytic system, they boosted its ability to work selectively on carbon-fluorine bonds.
“We have shown that this reaction is a very useful tool for sequentially changing parts of a molecule with great control,” says Group leader, Nobuaki Kambe. “Our control over the chemistry of carbon-fluorine bonds should enable much more synthetic freedom for building complex carbon structures.”
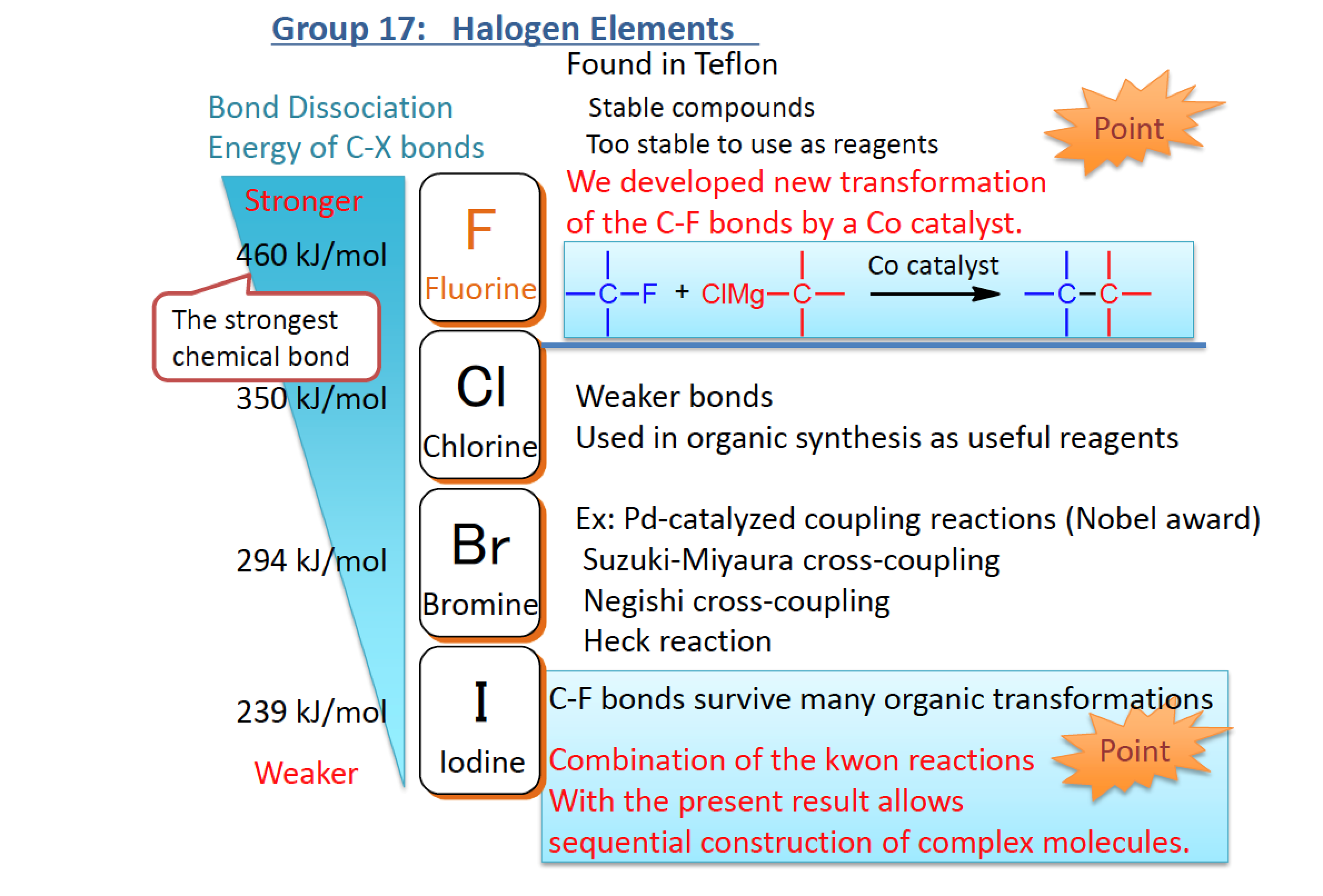
Figure 1. Outline of this research. (Osaka University)

Figure 2. Co-catalyzed cross-coupling of alkyl fluorides with alkyl Grignard reagents. (Osaka University)
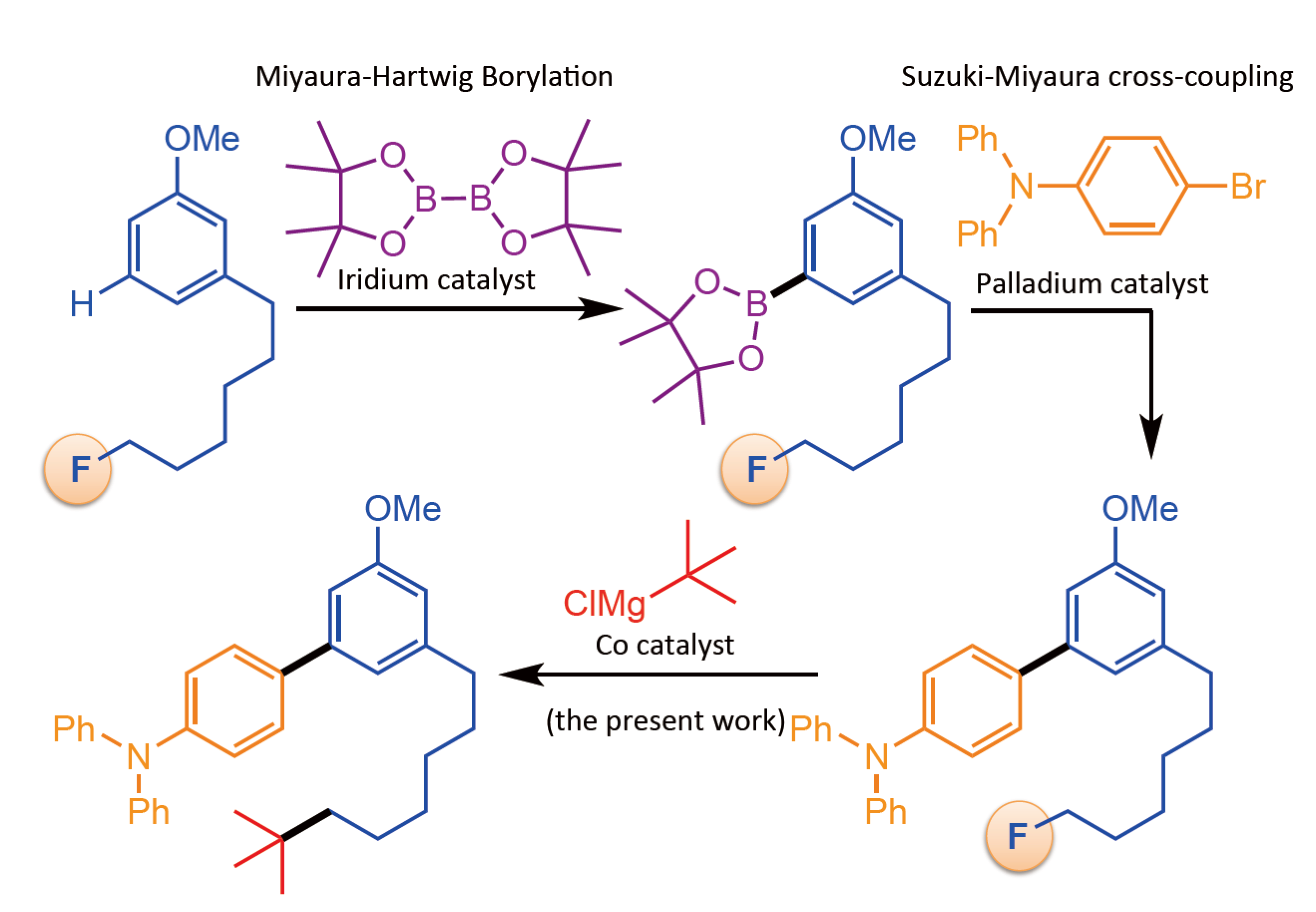
Figure 3. Iterative coupling reaction using the present catalytic system. (Osaka University)
To learn more about this research, please view the full research report entitled " Co-Catalyzed Cross-Coupling Reaction of Alkyl Fluorides with Alkyl Grignard Reagents " at this page of Organic Letters.
Related link
