Elucidation of Mechanism Behind the Decline of Dynamic Vision in the Elderly
Provides insight into the mechanism and importance of set location of synapse formation in nerve cells
A group of researchers led by Professor FURUKAWA Takahisa (Professor, Laboratory for Molecular and Developmental Biology, Institute for Protein Research, Osaka University) and SANUKI Rikako (Assistant Professor, Laboratory for Molecular and Developmental Biology, Institute of Protein Research, Osaka University) have elucidated the mechanism in which synapses are formed on retinal visual cells, as well as their indispensability to dynamic vision. These results have elucidated the significance of the formation of nerve cell synapses in given positions on the neural circuit, which will lead to a better understanding of the mechanism responsible for the decline in visual ability and corresponding decline in driving ability in elderly drivers.
In vertebrate retinal development, the axonal terminals of retinal neurons make synaptic contacts within narrow fixed regions, and these locations are maintained thereafter. However, the mechanisms and biological logic of the organization of these fixed synapse locations are poorly understood. We show here that a membrane scaffold protein, 4.1G, is highly expressed in retinal photoreceptors and is essential for the arrangement of their correct synapse location. The 4.1G-deficient retina exhibits mislocalization of photoreceptor terminals, although their synaptic connections are normally formed. The 4.1G protein binds to the AP3B2 protein, which is involved in neuronal membrane trafficking, and promotes neurite extension in an AP3B2-dependent manner. 4.1G mutant mice showed visual acuity impairments in an optokinetic response, suggesting that correct synapse location is required for normal visual function. Taken together, the data in this study provide insight into the mechanism and importance of proper synapse location in neural circuit formation.

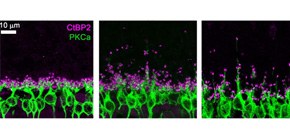
Figure 1. Ectopic synapse formation in the 4.1G-deficient and aged wild-type retinas.
A. Immunostaining images of photoreceptor synapses (magenta) and bipolar cells (green). The aged wild-type retina and the 4.1G-deficient retina exhibited ectopic synapse positions compared with the young wild-type retina.
B. A schematic model of synapse positions of wild-type and 4.1G deficient retinas. While the wild-type retina develops synapses within the outer plexiform layer, the 4.1G-deficient retina shows ectopic synapses.

Figure 2. Visual acuity test of 4.1G-deficient mice. Visual acuity of wild-type and 4.1G-deficient mice were measured by opto-kinetic responses. Spatial and contrast sensitivities on moving stripes in 4.1G-deficient mice were impaired.
Key words
- 4.1G protein : 4.1G protein is membrane-cytoskeletal protein, which anchors a transmembrane protein to the cytoskeleton.
- Photoreceptor cell : A photoreceptive neuron which converts light to electrical signal in the retina.
- Visual acuity : Mouse vision is measured by visual responses to moving stripes and is quantified by spatial frequencies and contrast sensitivities.
- Outer plexiform layer : A retinal layer where synaptic connections are formed among photoreceptor, bipolar, and horizontal cells.
To learn more about this research, please view the full research report entitled " Protein-4.1G-Mediated Membrane Trafficking Is Essential for Correct Rod Synaptic Location in the Retina and for Normal Visual Function " at this page of the Cell Reports website.
Related link
