Fe42 Magnetic Cage Nano-cluster Molecule Successfully Synthesized
Collaborative Research Records World’s Hugest Ground-State Spin Quantum Number (S = 45)
Collaborative research by Kyushu University, Dalian University of Technology, Japan Synchrotron Radiation Research Institute (JASRI), Kumamoto University, Kyushu Institute of Technology, Osaka University, and Tohoku University yielded a magnetic cage nano-cluster molecule, recording the world's hugest ground-state spin quantum number (S = 45). This molecule was structurally identified at SPring-8 (JASRI) and magnetically characterized at Pulsed High Magnetic Field Facility (Tohoku University).
Nowadays, competitive research on an international level is conducted in the field of molecular magnetism, aiming to artificially create magnetic molecules with huge spin as nanoscale permanent magnets. The collaboration group succeeded in setting the world record for molecular magnetization (90 Bohr magnetons) by the precise molecular design of a nano-cluster molecule in which ferromagnetic interactions are effective between eighteen paramagnetic iron atoms on the molecule. At the same time, this molecule is interesting in how its hollow cage shape is capable of accommodating other molecules, acting as a molecular flask.
Fig. 1.
a) Photo of cubic crystals consisting of Fe 42 nano cluster molecules.
b) Molecular structure of an Fe 42 nano cluster molecule clarified at the Beamline BL02B1/SPring-8 for single crystal structure analysis.
(Divalent and trivalent iron atoms are shown with green and orange spheres, respectively.) The iron-atom network forms a hollow cage (blue sphere with a diameter of 1.5 nanometers) capable of accommodating other chemical species. 1 nanometer is 1 part per billion meter (0.000000001m).
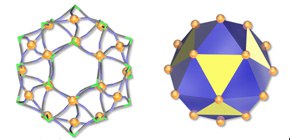
Fig. 2
A schematic view of the hollow polyhedron formed by eighteen trivalent iron atoms (orange spheres) bearing magnetism. All face-center positions of square faces in the yellow cuboctahedron are capped to blue-colored stellation, showing beautiful high symmetry of molecular shape.
Fig. 3
Magnetic characteristics of the Fe 42 nano cluster molecule and schematic arrangement of atomic magnets. Upward arrows stand for atomic magnets.
Red circles are experimental values and the black curve is a theoretical prediction, showing a good agreement.
To learn more about this research, please view the full research report entitled " A Ferromagnetically Coupled Fe 42 Cyanide-Bridged Nanocage " at this page of the Nature Communication website.
Related Links
