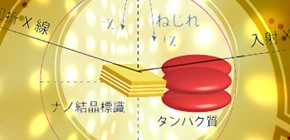
Intramolecular motion in protein molecules successfully monitored by Diffracted X-ray Tracking (DXT)
In molecular designing for drug development, it is important to quantitatively evaluate the molecular motions of proteins and detect to a high degree of accuracy differences in motion between normal proteins and abnormal proteins. A group of researchers led by Professor Yuji SASAKI at the Graduate School of Frontier Sciences, The University of Tokyo, developed Diffracted X-ray Tracking (DXT) capable of tracking intramolecular motion of proteins. However, with this DXT method, the range capable of detecting angular changes in intramolecular structure was quite limited making it difficult to obtain statistical data on molecular motion.
By improving the DXT method, a group led by Professor SASAKI -- Kohei ICHIYANAGI , Assistant professor, Sasaki Lab., Dr. Hiroshi SEKIGUCHI , Japan Synchrotron Radiation Research Institute, Professor Yoshihisa INOUE , Division of Applied Chemistry, Graduate School of Engineering, Osaka University -- succeeded in obtaining statistical data on the tilting and twisting motions of single protein molecules occurring over a short period of time. This new DXT method using hard X-rays with a wide energy bandwidth from the photon source has enabled researchers to measure complex tilting and twisting motions of protein molecules in a wide angular range over a short period of time. Installing a single toroidal X-ray mirror to BL28B2 beamline at the radiation facility SPring-8, this group succeeded in measuring a wide angular range from 2.4 to 22.6.
This group's achievement demonstrates that the wide angle DXT method is a practical means for measuring individual molecular motion at any radiation facility in the world. Quantitative analysis of large-scale correlations regarding protein molecular functionality and intramolecular motion features will possibly promote the evaluation of beneficial and adverse effects in drug targets and the early detection of abnormal proteins.
Abstract
Diffracted X-ray tracking (DXT) enables the tilting and twisting motions of single protein molecules to be monitored with micro- to milliradian resolution using a highly brilliant X-ray source with a wide energy bandwidth. We have developed a technique to monitor single molecules using gold nanocrystals attached to individual protein molecules using the BL28B2 beamline at SPring-8. In this paper we present the installation of a single toroidal X-ray mirror at BL28B2 to focus X-rays in an energy range of 10–20 keV (△E/E = 82% for an X-ray with a wide energy bandwidth). With this beamline we tracked diffraction spots from gold nanocrystals over a wide angle range than that using quasi-monochromatic X-rays. Application of the wide angle DXT technique to biological systems enabled us to observe the on-site motions of single protein molecules that have been functionalized in vivo. We further extend the capability of DXT by observing the fractional tilting and twisting motions of inner proteins under various conditions. As a proof of this methodology and to determine instrumental performance the intramolecular motions of a human serum albumin complex with 2-anthracenecarboxylic acid was investigated using the BL28B2 beamline. The random tilting and twisting intramolecular motions are shown to be directly linked to the movement of individual protein molecules in the buffer solution.

Figure 1

Figure 2

Figure 3

Figure 4
To learn more about this research, please read the full research report entitled " Diffracted X-ray Tracking (DXT) for Monitoring Intramolecular Motion in Individual Protein Molecules using broad band X-ray " at this page of the Review of Scientific Instruments website.
Related link :
