Visualization of the behavior of sugar transport proteins
Mechanism for developing type II diabetes elucidated
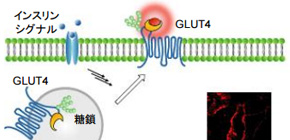
A group of researchers led by Professor KIKUCHI Kazuya at the Graduate School of Engineering, Osaka University, clarified the role of a N-glycan chain on glucose transporter type 4 (GLUT4) by developing a method for visualizing intracellular trafficking of proteins.
The GLUT4 translocation disorder is known to be associated with the onset of type II diabetes. GLUT4 is translocated to the cell membrane in response to insulin stimulation and takes up glucose in blood, decreasing blood sugar levels. The role of the N-glycan chain on GLUT4 in intracellular trafficking has drawn attention in recent years.
The fluorogenic probe developed by this group has the function of increasing fluorescence intensity by binding to a protein. Thanks to the cell-impermeability of the fluorogenic probe, it’s possible to label only proteins that appear on the cell membrane. These functions have made it possible to quickly fluorescence-label only GLUT4 that is translocated to the cell membrane by fluorogenic probes and clearly detect fluorescence of GLUT4 translocation to the cell membrane.
Previously, kinetic analysis of GLUT4 was performed by using fluorescence proteins and an immunostaining method; however, it was impossible to precisely examine if GLUT4 in the cell had been transiently translocated to the cell membrane.
The technique developed by this group has made it possible to precisely determine the translocation progress of GLUT4; that is, it has become possible to record the evidence showing that GLUT4 was transiently translocated to the cell membrane. It was clarified that GLUT4 with abnormalities in the N-glycan chain was transiently translocated to the cell membrane but was rapidly internalized without retention on the cell membrane, and that the N-glycan chain played a role in retaining GLUT4 on the cell membrane.
This group’s achievement will lead to the clarification of the mechanism behind the localization of GLUT4 and the mechanism for developing diabetes, as well as the development of new types of treatment drugs.
Abstract
Glucose transporter 4 (GLUT4) is an N-glycosylated protein that maintains glucose homeostasis by regulating the protein translocation. To date, it has been unclear whether the N-glycan of GLUT4 contributes to its intracellular trafficking. Here, to clarify the role of the N-glycan, we developed fluorogenic probes that label cytoplasmic and plasma-membrane proteins for multicolor imaging of GLUT4 translocation. One of the probes, which is cell impermeant, selectively detected exocytosed GLUT4. Using this probe, we verified the 'log' of the trafficking, in which N-glycan-deficient GLUT4 was transiently translocated to the cell membrane upon insulin stimulation and was rapidly internalized without retention on the cell membrane. The results strongly suggest that the N-glycan functions in the retention of GLUT4 on the cell membrane. This study showed the utility of the fluorogenic probes and indicated that this imaging tool will be applicable for research on various membrane proteins that show dynamic changes in localization.
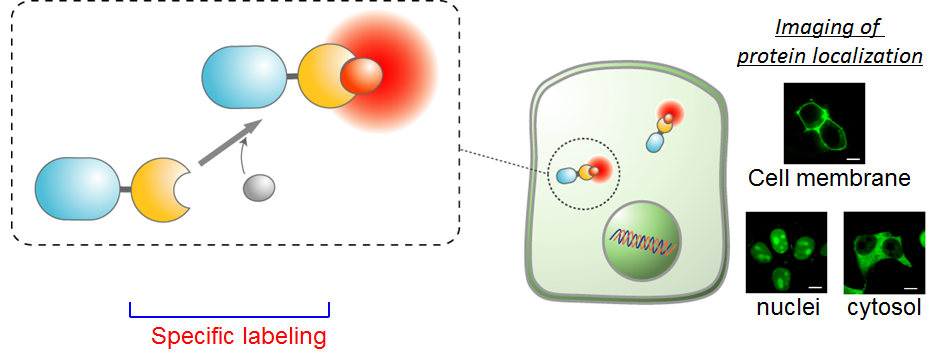
Figure 1
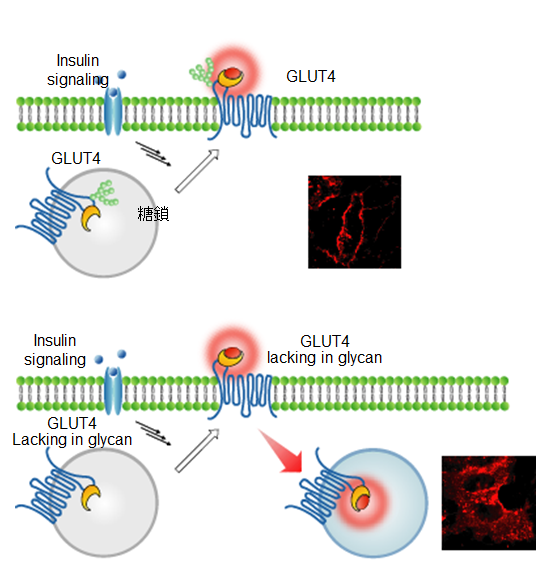
Figure 2
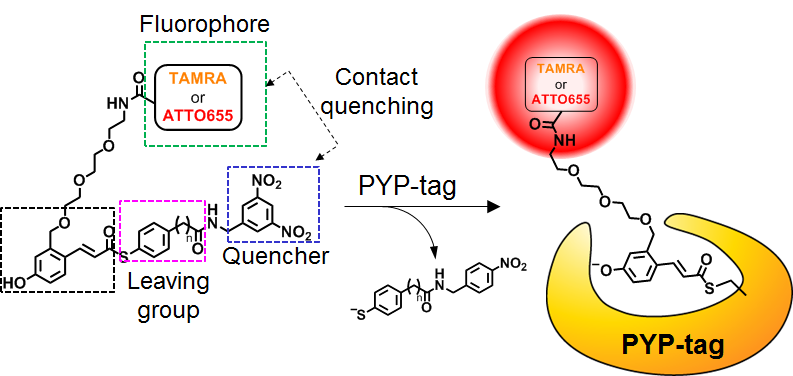
Figure 3
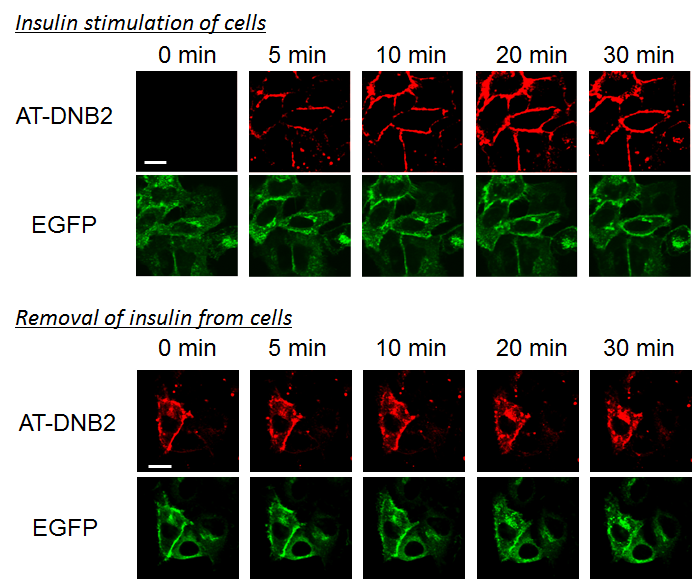
Figure 4
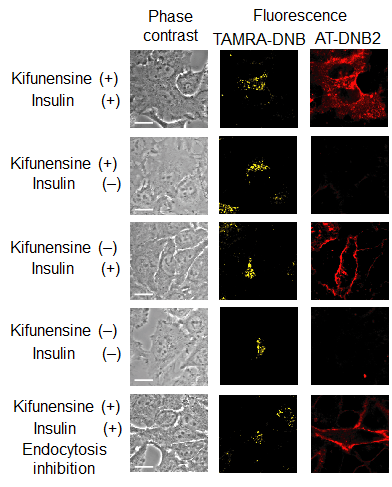
Figure 5
To learn more about this research, please view the full research report entitled “ Fluorogenic Probes Reveal a Role of GLUT4 N-Glycosylation in Intracellular Trafficking ” at this page of the Nature Chemical Biology website.
Related links
