Visualized the moment UV-DDB protein repairs UV damage on the genome within cells
Elucidating the pathogenesis mechanism of xeroderma pigmentosum (XP)
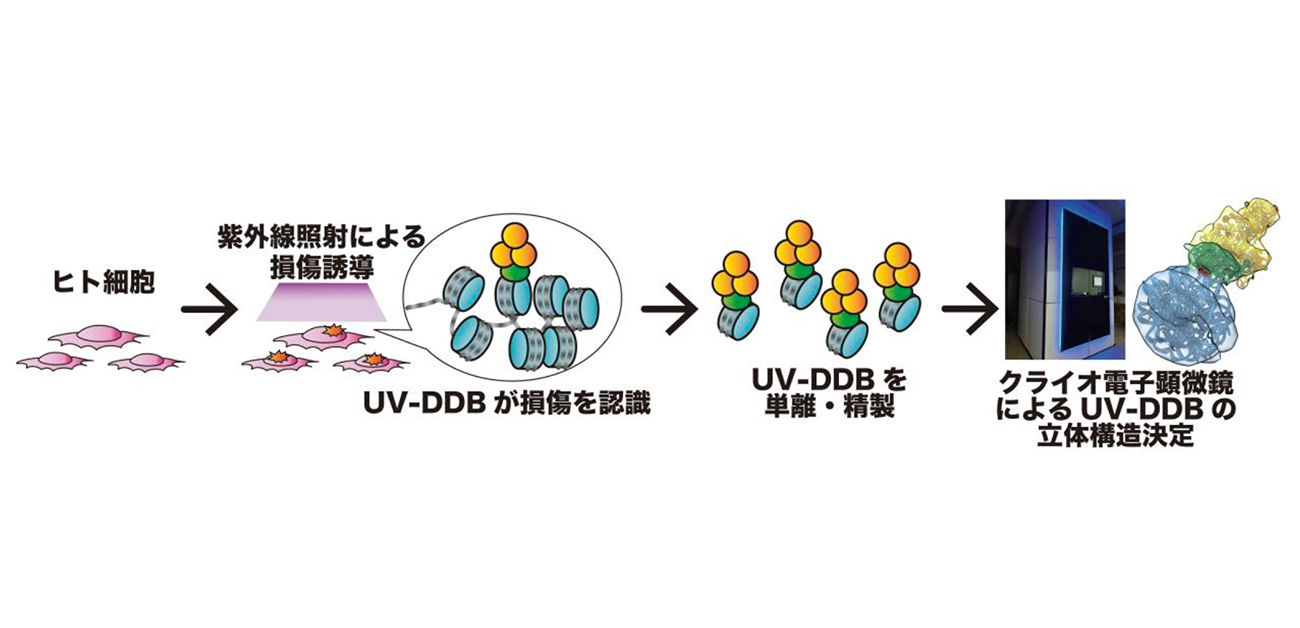
- By isolating a DNA repair protein (UV-DDB protein) that binds to UV damage in genomic DNA from cells and analyzing it with a cryo-electron microscopy (ChIP-CryoEM), it has succeeded in visualizing the process of UV damage repair within cells.
- Until now, many aspects of the repair process for UV damage to genomic DNA are unknown, making it difficult to directly observe the process within cells. This research has established a new technological foundation for elucidating the repair process within cells.
- The UV-DDB protein targeted in this study is known to be the causative factor of the intractable disease xeroderma pigmentosum. As there is currently no fundamental treatment for this disease, it is expected that the results of this study will lead to the development of future therapies.
Overview of UV-DDB protein visualization by ChIP-CryoEM analysis
Credit: Junpei Yamamoto
Outlines
A research group including Assistant Professor Shota Matsumoto and Professor Hitoshi Kurumizaka at the Institute of Molecular and Cellular Biosciences (IMCB) of the University of Tokyo, Professor Kaoru Sugasawa at the Biosignal Research Center of Kobe University, and Distinguished Honorary Professor
Shigenori Iwai and Associate Professor Junpei Yamamoto of the Graduate School of Engineering Science, the University of Osaka, has succeeded in visualizing the three-dimensional structure of the UV-DDB protein complex during repair process of UV damage to genomic DNA within cells.
In this study, Professor Kurumizaka and his team applied their proprietary technology for visualizing intracellular proteins called ChIP-CryoEM, which revealed for the first time the structure of UV-DDB protein, known as a DNA repair protein, bound to UV-damaged DNA. Unlike previous studies, the novelty lies in the direct isolation of UV-DDB proteins from within cells and the determination of their structure. This is expected to contribute to understanding and developing treatments for intractable genetic diseases caused by DNA repair defects, including xeroderma pigmentosum.
Research Contents
The UV-DDB protein was previously known to be the causative factor of xeroderma pigmentosum and to play an important role in repairing UV damage to genomic DNA and other tissues. Genomic DNA is wrapped around histones within cells to form chromatin structures, and many aspects remain unknown about how it repairs UV damage within this complex structural environment (Fig. 1).
Fig. 1 Chromatin structure formed by genomic DNA within cells
Credit: Junpei Yamamoto
The research team attempted to elucidate the intracellular DNA repair mechanism and applied their unique technology ChIP-CryoEM, which combines chromatin immunoprecipitation (ChIP) and cryo-electron microscopy (Cryo-EM), to UV-DDB proteins. As a result, the research team directly isolated the UV-DDB complex bound to UV damage on the nucleosome, and succeeded for the first time in elucidating its three-dimensional structure. This revealed that UV-DDB protein directly recognizes and binds to DNA damage on nucleosomes, even in the structurally complex environment of nucleosomes, with almost no steric inhibition by histones (Fig. 2).
Fig. 2 Three-dimensional structure of UV-DDB protein bound to UV damaged on nucleosomes in cells visualized by ChIP-CryoEM
Credit: Junpei Yamamoto
Furthermore, the research team performed high-resolution observations of the binding process of UV-DDB protein to UV damage, revealing for the first time the detailed binding structure to cyclobutane pyrimidine dimers (CPDs), a typical type of UV damage. Two amino acid residues in the UV-DDB protein were found to interact specifically with the CPD damage, allowing it to directly recognize and bind to the CPD damage on the nucleosome. This has elucidated the molecular foundation of how UV damage is recognized and repaired within cells (Fig. 3).
Fig. 3 Detailed three-dimensional structure of UV-DDB bound to UV-damaged CPD
Credit: Junpei Yamamoto
The findings have potential applications not only to research on UV damage but also to research on DNA damage repair caused by ionizing radiation and toxic substances and are expected to make a significant contribution to understanding the repair mechanisms of genomic DNA as a whole. Abnormalities in DNA repair mechanisms are a major cause of intractable genetic diseases such as xeroderma pigmentosum, and the findings of this research may significantly contribute to elucidating their molecular foundation and developing therapeutic approaches. Through these efforts, it is desired that this achievement will be an important and groundbreaking step towards overcoming intractable diseases.
Notes
The article, “Structural basis of cyclobutane pyrimidine dimer recognition by UV-DDB in the nucleosome,” was published in British Journal of Scientific Reports at DOI: 10.1038/s41467-025-65486-5
