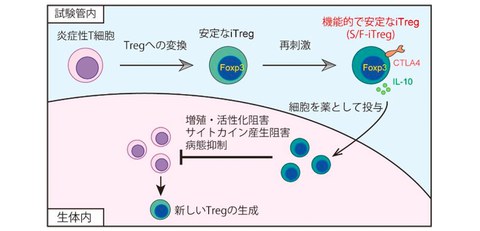
A method for producing stable and functional artificial regulatory T (Treg) cells "S/F-iTreg" is developed
Aiming to treat autoimmune and other inflammatory diseases
- Aiming to treat inflammatory diseases with regulatory T (Treg) cells, a method to artificially induce functional and stable Tregs (S/F-iTregs) from T cells that cause inflammation was developed.
- Until now, artificial Tregs (iTregs) have had issues with stability and functionality, but a new production method that improves the culture method has succeeded in providing them with the same functions and properties as naturally occurring Tregs (Natural Tregs; nTregs).
- Administration of S/F-iTregs improved symptoms in colitis and GvHD model mice. It is also confirmed that S/F-iTregs can be induced from T cells of patients with human autoimmune diseases such as Crohn's disease and SLE.
- The use of S/F-iTregs as a biopharmaceutical is expected to realize new antigen-specific treatments that specifically suppress reactions that cause autoimmune and inflammatory diseases, as well as the induction of long-term tolerance.
Outlines
A research group including Specially Appointed Associate Professor (Full-time) Morihisa Mikami and Specially Appointed Professor (Full-time) Shimon Sakaguchi of the Immunology Frontier Research Center at the University of Osaka has developed a method for artificially inducing functional and stable regulatory T (Treg) cells from inflammation-causing T cells using a special culture method (Fig. 1).
Tregs are special T cells with immunosuppressive capabilities and are expected to contribute to the treatment of autoimmune and inflammatory diseases. To realize this treatment, utilizing artificially induced Tregs (iTregs) to compensate for the limitations of Tregs (nTregs) that exist naturally in the body is attracting attention. While there are expectations for realizing antigen-specific immunosuppression, there have been issues with the stability and functionality of the cells.
In this study, by improving the culture method, the research group developed a new method for artificially inducing Tregs using disease-causing T cells as a raw material. This new method has succeeded in creating Tregs with properties and abilities closer to those of naturally occurring Tregs than conventional artificial Tregs, making it possible to use functional and stable Tregs (S/F-iTregs) in cell therapy.
The S/F-iTregs maintain a stable state as Tregs even when administered into the body, and only react to specific antigens to suppress immune responses. When S/F-iTregs were administered to model mice of inflammation such as colitis and GvHD, it is found that the inflammatory response was suppressed for a long period of time. In addition, experiments using human cells also revealed that disease-causing T cells collected from patients with autoimmune diseases such as Crohn's disease and SLE could be induced to become S/F-iTregs.
The results of this study suggest that the use of S/F-iTregs as biopharmaceutical may realize new antigen-specific treatments that specifically suppress reactions that cause autoimmune and inflammatory diseases, as well as the induction of long-term tolerance.
Fig. 1 An induction method for new Treg formulation
Functional and stable Tregs (S/F-iTregs) are artificially induced from inflammatory T cells and used for treatment.
Credit: Norihisa Mikami
Research Background
Clinical research and trials for Treg transfer therapy are already underway, but many practical issues still exist. The widely used method today involves collecting naturally occurring Tregs (nTregs) from the living body, stimulating them to proliferate in vitro, and then re-administering them. However, issues such as the scarcity of nTreg cells as a raw material, stability during culture, survival after introduction, antigen specificity, and therapeutic efficacy remain.
On the other hand, cell therapy using artificially induced Tregs (iTregs) has the advantage of being able to produce large amounts of antigen-specific Treg cells. The therapeutic concept of converting inflammatory T cells into iTregs and using them as a biopharmaceutical to control the immune system is a promising candidate for realizing antigen-specific immunosuppression. This method converts disease-causing activated T cells into iTregs while maintaining their reactivity, which is expected to result in antigen-specific suppression. However, because it is an artificial induction, there have been many problems with the properties of Tregs, and until now, their stability and functionality have been insufficient.
Research Contents
The research group succeeded in providing artificially induced iTregs with properties similar to those of nTregs by combining multiple innovations and improvements to the culture method. These iTregs are stable and highly functional, so have been named Stable/Functional iTregs (S/F-iTregs).
In order to precisely compare the properties of S/F-iTregs, the researchers performed RNA sequencing (RNA-seq) analysis alongside nTregs and conventional iTregs. The results clarified that unlike normal T cells and normal iTregs, S/F-iTregs showed a gene expression pattern similar to that of in vivo nTregs (Fig. 2). When the suppressive functions were actually measured in vitro, both S/F-iTregs and nTregs had similar levels of suppressive activity.
The research group also purified inflammatory T cells from diseased mice and reviewed them to be used as a raw material to induce S/F-iTregs. Using conventional methods, it is extremely difficult to induce Tregs from such inflammatory T cells, but by using the S/F-iTreg production method, the research team has succeeded in inducing functional iTregs efficiently. Experiments in which S/F-iTregs were induced by purified T cells that react to specific antigens have also shown that the induced S/F-iTregs specifically and efficiently suppress reactions to the particular antigens. These results suggest that by inducing Tregs while retaining the reaction of disease-causing T cells, it is possible to create Tregs that specifically and effectively suppress the reactions that cause the disease.
The research group administered S/F-iTreg to colitis and GvHD model mice to examine the therapeutic effects. When colitis was induced in mice, administration of S/F-iTregs suppressed the weight loss associated with colitis for more than six weeks (Fig. 3A). Similarly, in a GvHD model mice, administration of S/F-iTregs extended their survival (Fig. 3B). It has also been confirmed that Tregs effectively suppress inflammatory responses in these mice, such as by suppressing cytokine production from T cells.
Finally, the researchers confirmed whether S/F-iTregs could be induced from human T cells. T cells from the blood of patients with autoimmune diseases such as Crohn's disease and SLE were purified and used a raw material to induce S/F-iTregs. As a result, they succeeded in converting them into S/F-iTregs at a high rate. It is also confirmed that these S/F-iTregs were also confirmed to have the effect of suppressing the proliferation of inflammatory T cells in the same patients in vitro.
Fig. 2 Gene expression patterns of S/F-iTregs
Cellular gene expression levels were assessed by RNA sequencing (RNA-seq). Higher red proportion of the heatmap indicates higher gene expression. While normal iTregs express low levels of genes (such as Foxp3) that are highly expressed in nTregs, S/F-iTregs show a gene expression pattern similar to that of nTregs.
Credit: Norihisa Mikami
Fig. 3 Suppressive effect of S/F-iTreg on inflammation model mice
(A) S/F-iTregs were administered to colitis model mice and measured their body weight for six weeks.
(B) S/F-iTregs were administered to a GvHD model mice and observed their survival for 60 days.
Credit: Norihisa Mikami
Social Impact of the Research
This research has developed a new method for producing functional and stable artificial regulatory T (Treg) cells using inflammatory T cells involved in the development of autoimmune and inflammatory diseases as raw materials. It is expected that using these S/F-iTregs as biopharmaceutical will provide antigen-specific and effective immunotherapy for autoimmune and inflammatory diseases, transplant rejection, and GvHD.
Notes
The article, “Generating functionally stable and antigen-specific Treg cells from effector T cells for cell therapy of inflammatory diseases,” was published in Science Translational Medicine at
